Liver fat quantification: where do we stand?
- PMID: 33025153
- PMCID: PMC7700836
- DOI: 10.1007/s00261-020-02783-1
Liver fat quantification: where do we stand?
Abstract
Excessive intracellular accumulation of triglycerides in the liver, or hepatic steatosis, is a highly prevalent condition affecting approximately one billion people worldwide. In the absence of secondary cause, the term nonalcoholic fatty liver disease (NAFLD) is used. Hepatic steatosis may progress into nonalcoholic steatohepatitis, the more aggressive form of NAFLD, associated with hepatic complications such as fibrosis, liver failure and hepatocellular carcinoma. Hepatic steatosis is associated with metabolic syndrome, cardiovascular disease and represents an independent risk factor for type 2 diabetes, cardiovascular disease and malignancy. Percutaneous liver biopsy is the current reference standard for NAFLD assessment; however, it is an invasive procedure associated with complications and suffers from high sampling variability, impractical for clinical routine and drug efficiency studies. Therefore, noninvasive imaging methods are increasingly used for the diagnosis and monitoring of NAFLD. Among the methods quantifying liver fat, chemical-shift-encoded MRI (CSE-MRI)-based proton density fat-fraction (PDFF) has shown the most promise. MRI-PDFF is increasingly accepted as quantitative imaging biomarker of liver fat that is transforming daily clinical practice and influencing the development of new treatments for NAFLD. Furthermore, CT is an important imaging method for detection of incidental steatosis, and the practical advantages of quantitative ultrasound hold great promise for the future. Understanding the disease burden of NAFLD and the role of imaging may initiate important interventions aimed at avoiding the hepatic and extrahepatic complications of NAFLD. This article reviews clinical burden of NAFLD, and the role of noninvasive imaging techniques for quantification of liver fat.
Keywords: Hepatic steatosis; Liver fat quantification; Magnetic resonance imaging; NAFLD; NASH; Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Noninvasive quantitative biomarker.
Conflict of interest statement
Conflicts of interest:
No authors have any relevant conflicts. Unrelated to this work, Dr. Reeder consults with HeartVista and has ownership interests in Calimetrix, Reveal Pharmaceuticals, Cellectar Biosciences, and Elucent Medical.
Figures

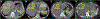


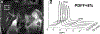


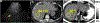
References
-
- Chalasani N, Younossi Z, Lavine JE, et al. (2018) The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases. 67:30 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

