Early relapse rate determines further relapse risk: results of a 5-year follow-up study on pediatric CFH-Ab HUS
- PMID: 33025207
- PMCID: PMC7910231
- DOI: 10.1007/s00467-020-04751-9
Early relapse rate determines further relapse risk: results of a 5-year follow-up study on pediatric CFH-Ab HUS
Abstract
Background: The complement factor H antibody (CFH-Ab)-associated hemolytic uremic syndrome (HUS) forms a distinct subgroup within the complement-mediated HUS disease spectrum. The autoimmune nature of this HUS subgroup implies the potential benefit of a targeted immunosuppressive therapy. Data on long-term outcome are scarce.
Methods: This observational study evaluates the clinical outcome of 19 pediatric CFH-Ab HUS patients from disease onset until their 5-year follow-up.
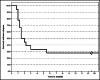
Results: All but one relapse occurred during the first 2 years, and patients who had no relapse within the first 6 months were relapse-free until the end of the observation period. Kidney function at disease onset determines long-term kidney function: all individuals with normal kidney function at disease onset had normal kidney function after 5 years, and all patients with reduced kidney function at onset had impaired kidney function at the last follow-up. Level of CFH-Ab titer at disease onset was not correlated with a higher risk of recurrences or worse long-term outcome after 5 years. Resolution of CFH-Ab titers after 5 years was common.
Conclusions: CFH-Ab HUS patients have a varied overall long-term course. Early relapses are common, making close surveillance during the first years essential, regardless of the initial CFH-Ab titer.
Keywords: CFH-Ab; Hemolytic uremic syndrome; Immunosuppressive therapy.
Conflict of interest statement
J.H., M.R. K., and R.W. have received honoraria from Alexion Pharmaceuticals Inc. and/or unrestricted educational grants and have served on various advisory boards.
Figures


References
-
- Hofer J, Janecke AR, Zimmerhackl LB, Riedl M, Rosales A, Giner T, Cortina G, Haindl CJ, Petzelberger B, Pawlik M, Jeller V, Vester U, Gadner B, van Husen M, Moritz ML, Würzner R, Jungraithmayr T, German-Austrian HUS Study Group Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2013;8:407–415. doi: 10.2215/CJN.01260212. - DOI - PMC - PubMed
-
- Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115:379–387. doi: 10.1182/blood-2009-05-221549. - DOI - PMC - PubMed
-
- Geerdink LM, Westra D, van Wijk JA, Dorresteijn EM, Lilien MR, Davin JC, Kömoff M, Van Hoeck K, van der Vlugt A, van den Heuvel LP, van de Kar NC. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27:1283–1291. doi: 10.1007/s00467-012-2131-y. - DOI - PMC - PubMed
-
- Sinha A, Gulati A, Saini S, Blanc C, Gupta A, Gurjar BS, Saini H, Kotresh ST, Ali U, Bhatia D, Ohri A, Kumar M, Agarwal I, Gulati S, Anand K, Vijayakumar M, Sinha R, Sethi S, Salmona M, George A, Bal V, Singh G, Dinda AK, Hari P, Rath S, Dragon-Durey MA, Bagga A. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014;85:1151–1160. doi: 10.1038/ki.2013.373. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

