Recent advances in the nucleic acid-based diagnostic tool for coronavirus
- PMID: 33025503
- PMCID: PMC7538041
- DOI: 10.1007/s11033-020-05889-3
Recent advances in the nucleic acid-based diagnostic tool for coronavirus
Abstract
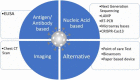
Recently in China, a novel coronavirus outbreak took place which caused pneumonia-like symptoms. This coronavirus belongs to the family of SARS and MERS and causes respiratory system disease known as COVID-19. At present we use polymerase chain reaction (PCR) based molecular biology methods for the detection of coronavirus. Other than these PCR based methods, some improved methods also exist such as microarray-based techniques, Real time-quantitative PCR, CRISPR-Cas13 based tools but almost all of the available methods have advantages and disadvantages. There are many limitations associated with this method and hence there is a need for a fast, more sensitive, and specific diagnostic tool which can detect a greater number of samples in less time. Here we have summarised currently available nucleic acid-based diagnostic methods for the detection of coronavirus and the need for developing a better technique for a fast and sensitive detection of coronavirus infections. Nucleic acid based detection tool for SARS-CoV-2.
Keywords: Amplification; COVID-19; Coronavirus; Microarray; Nucleic acid detection; qRT-PCR.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19.Med Hypotheses. 2020 Aug;141:109786. doi: 10.1016/j.mehy.2020.109786. Epub 2020 Apr 25. Med Hypotheses. 2020. PMID: 32361529 Free PMC article.
-
A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2).Virology. 2020 Oct;549:1-4. doi: 10.1016/j.virol.2020.07.006. Epub 2020 Jul 29. Virology. 2020. PMID: 32758712 Free PMC article.
-
Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing.N Engl J Med. 2020 Oct 8;383(15):1492-1494. doi: 10.1056/NEJMc2026172. Epub 2020 Sep 16. N Engl J Med. 2020. PMID: 32937062 Free PMC article. No abstract available.
-
[Laboratory testing techniques for SARS-CoV-2].Nan Fang Yi Ke Da Xue Xue Bao. 2020 Feb 29;40(2):164-167. doi: 10.12122/j.issn.1673-4254.2020.02.04. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32376546 Free PMC article. Review. Chinese.
-
Recent advances in methods for the diagnosis of Corona Virus Disease 2019.J Clin Lab Anal. 2022 Jan;36(1):e24178. doi: 10.1002/jcla.24178. Epub 2021 Dec 17. J Clin Lab Anal. 2022. PMID: 34921443 Free PMC article. Review.
Cited by
-
Aspects of Nanotechnology for COVID-19 Vaccine Development and Its Delivery Applications.Pharmaceutics. 2023 Jan 30;15(2):451. doi: 10.3390/pharmaceutics15020451. Pharmaceutics. 2023. PMID: 36839773 Free PMC article. Review.
-
A collection of the novel coronavirus (COVID-19) detection assays, issues, and challenges.Heliyon. 2021 Jun;7(6):e07247. doi: 10.1016/j.heliyon.2021.e07247. Epub 2021 Jun 6. Heliyon. 2021. PMID: 34124407 Free PMC article. Review.
-
SARS-CoV-2 Subgenomic N (sgN) Transcripts in Oro-Nasopharyngeal Swabs Correlate with the Highest Viral Load, as Evaluated by Five Different Molecular Methods.Diagnostics (Basel). 2021 Feb 12;11(2):288. doi: 10.3390/diagnostics11020288. Diagnostics (Basel). 2021. PMID: 33673182 Free PMC article.
-
Comparative study of different SARS-CoV-2 diagnostic techniques.J Virol Methods. 2021 Dec;298:114281. doi: 10.1016/j.jviromet.2021.114281. Epub 2021 Sep 13. J Virol Methods. 2021. PMID: 34530011 Free PMC article.
-
Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms.Cells. 2022 Mar 31;11(7):1182. doi: 10.3390/cells11071182. Cells. 2022. PMID: 35406746 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous