Multifaceted Beneficial Effects of Erdosteine: More than a Mucolytic Agent
- PMID: 33025535
- PMCID: PMC7647991
- DOI: 10.1007/s40265-020-01412-x
Multifaceted Beneficial Effects of Erdosteine: More than a Mucolytic Agent
Abstract
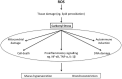
Erdosteine is a drug approved for the treatment of acute and chronic pulmonary diseases, originally developed as a mucolytic agent. It belongs to the thiol-based family of drugs that are known to also possess potentially important antioxidant and anti-inflammatory properties, and exhibit antibacterial activity against a variety of medically important bacterial species. Erdosteine is a prodrug that is metabolized to the ring-opening compound metabolite M1 (MET 1), which has mucolytic properties. Experimental studies have documented that erdosteine prevents or reduces lung tissue damage induced by oxidative stress and, in particular, that Met 1 also regulates reactive oxygen species production. The RESTORE study, which has been the only trial that investigated the effects of a thiol-based drug in chronic obstructive pulmonary disease (COPD) frequent exacerbators, documented that erdosteine significantly reduces the risk of acute exacerbations of COPD (AECOPDs), shortens their course, and also decreases the risk of hospitalization from COPD. The preventive action of erdosteine on AECOPDs was not affected by the presence or absence of inhaled corticosteroids (ICSs) or blood eosinophil count. These findings clearly contrast with the Global Initiative for Chronic Obstructive Lung Disease strategy's approach to use erdosteine only in those COPD patients not treated simultaneously with an ICS. Furthermore, they support the possibility of using erdosteine in a step-down approach that in COPD is characterized by the withdrawal of the ICS.
Conflict of interest statement
Mario Cazzola, Paola Rogliani, Luigino Calzetta and Clive Page are consultants to Recipharm, which manufactures erdosteine.
Figures



Similar articles
-
Use of thiols and implications for the use of inhaled corticosteroids in the presence of oxidative stress in COPD.Respir Res. 2023 Jul 31;24(1):194. doi: 10.1186/s12931-023-02500-8. Respir Res. 2023. PMID: 37517999 Free PMC article. Review.
-
Erdosteine: its relevance in COPD treatment.Expert Opin Drug Metab Toxicol. 2009 Mar;5(3):333-43. doi: 10.1517/17425250902814790. Expert Opin Drug Metab Toxicol. 2009. PMID: 19331595 Review.
-
Efficacy of erdosteine 900 versus 600 mg/day in reducing oxidative stress in patients with COPD exacerbations: Results of a double blind, placebo-controlled trial.Pulm Pharmacol Ther. 2015 Aug;33:47-51. doi: 10.1016/j.pupt.2015.06.004. Epub 2015 Jun 23. Pulm Pharmacol Ther. 2015. PMID: 26116425 Clinical Trial.
-
Use of mucolytics in COPD: A Delphi consensus study.Respir Med. 2020 Dec;175:106190. doi: 10.1016/j.rmed.2020.106190. Epub 2020 Nov 13. Respir Med. 2020. PMID: 33217537
-
Effect of Erdosteine on COPD Exacerbations in COPD Patients with Moderate Airflow Limitation.Int J Chron Obstruct Pulmon Dis. 2019 Dec 2;14:2733-2744. doi: 10.2147/COPD.S221852. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31819405 Free PMC article. Clinical Trial.
Cited by
-
Insights into Personalised Medicine in Bronchiectasis.J Pers Med. 2023 Jan 10;13(1):133. doi: 10.3390/jpm13010133. J Pers Med. 2023. PMID: 36675794 Free PMC article. Review.
-
Understanding the Role of the Antioxidant Drug Erdosteine and Its Active Metabolite on Staphylococcus aureus Methicillin Resistant Biofilm Formation.Antioxidants (Basel). 2021 Nov 29;10(12):1922. doi: 10.3390/antiox10121922. Antioxidants (Basel). 2021. PMID: 34943025 Free PMC article.
-
Use of Thiols in the Treatment of COVID-19: Current Evidence.Lung. 2021 Aug;199(4):335-343. doi: 10.1007/s00408-021-00465-3. Epub 2021 Aug 27. Lung. 2021. PMID: 34448938 Free PMC article. Review.
-
Anthrahydroquinone-2,6-Disulfonate Restores Lung Function in COPD Through Keap1/Nrf2 Pathway Activation.J Inflamm Res. 2025 Jul 18;18:9559-9579. doi: 10.2147/JIR.S524429. eCollection 2025. J Inflamm Res. 2025. PMID: 40697418 Free PMC article.
-
Use of thiols and implications for the use of inhaled corticosteroids in the presence of oxidative stress in COPD.Respir Res. 2023 Jul 31;24(1):194. doi: 10.1186/s12931-023-02500-8. Respir Res. 2023. PMID: 37517999 Free PMC article. Review.
References
-
- Dechant KL, Noble S. Erdosteine. Drugs. 1996;52(6):875–882. - PubMed
-
- Cazzola M, Calzetta L, Page C, Rogliani P, Matera MG. Thiol-based drugs in pulmonary medicine: much more than mucolytics. Trends Pharmacol Sci. 2019;40(7):452–463. - PubMed
-
- Miyake K, Kaise T, Hosoe H, Akuta K, Manabe H, Ohmori K. The effect of erdosteine and its active metabolite on reactive oxygen species production by inflammatory cells. Inflamm Res. 1999;48(4):205–209. - PubMed
-
- Hosoe H, Kaise T, Ohmori K, et al. Mucolytic and antitussive effects of erdosteine. J Pharm Pharmacol. 1999;51(8):959–966. - PubMed
-
- Hattori M. Mucociliary function of chronic inflammation in upper and lower airways. Auris Nasus Larynx. 1994;21(4):219–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

