TSNP: A two-stage nonparametric phase I/II clinical trial design for immunotherapy
- PMID: 33025762
- PMCID: PMC9386730
- DOI: 10.1002/pst.2075
TSNP: A two-stage nonparametric phase I/II clinical trial design for immunotherapy
Abstract
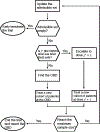
We develop a transparent and efficient two-stage nonparametric (TSNP) phase I/II clinical trial design to identify the optimal biological dose (OBD) of immunotherapy. We propose a nonparametric approach to derive the closed-form estimates of the joint toxicity-efficacy response probabilities under the monotonic increasing constraint for the toxicity outcomes. These estimates are then used to measure the immunotherapy's toxicity-efficacy profiles at each dose and guide the dose finding. The first stage of the design aims to explore the toxicity profile. The second stage aims to find the OBD, which can achieve the optimal therapeutic effect by considering both the toxicity and efficacy outcomes through a utility function. The closed-form estimates and concise dose-finding algorithm make the TSNP design appealing in practice. The simulation results show that the TSNP design yields superior operating characteristics than the existing Bayesian parametric designs. User-friendly computational software is freely available to facilitate the application of the proposed design to real trials. We provide comprehensive illustrations and examples about implementing the proposed design with associated software.
Keywords: adaptive design; immunotherapy; phase I/II clinical trial.
© 2020 John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

