MiR-27a Facilitates Breast Cancer Progression via GSK-3β
- PMID: 33025840
- PMCID: PMC7545786
- DOI: 10.1177/1533033820965576
MiR-27a Facilitates Breast Cancer Progression via GSK-3β
Abstract
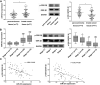
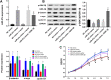
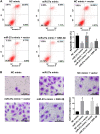
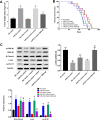
Breast cancer remains one of the leading causes of cancer-associated death in women. MiR-27a is highly expressed in breast cancer tissue. However, the underlying mechanisms that promote breast cancer progression are unknown. In this study, we investigated the regulatory mechanisms of miR-27a and its target glycogen Synthase Kinase 3-β (GSK-3β) in breast cancer cells. We found that miR-27a was highly expressed in breast cancer tissues, which downregulated GSK-3β expression. We further identified GSK-3β as a direct target of miR-27a, and found that the miR-27a mediated suppression of GSK-3β activated Wnt/β-catenin-associated proliferative and invasive factor in breast cancer. The cell transfection assay demonstrated the overexpression of miR-27a also enhanced cell proliferation and invasion, and reduced cell apoptosis through GSK-3β. Finally, we demonstrated that the overexpression of miR-27a facilitated breast cancer progression through its ability to down-regulate the phosphorylation of GSK-3β both in vivo and vitro. These findings highlighted miR-27a as a novel therapeutic target in breast cancer.
Keywords: GSK-3β; breast cancer; miR-27a; phosphorylation; therapeutic target.
Conflict of interest statement
Figures

Similar articles
-
In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/β-catenin signaling pathway.Oncotarget. 2017 Feb 28;8(9):15507-15519. doi: 10.18632/oncotarget.14662. Oncotarget. 2017. PMID: 28099945 Free PMC article.
-
Transcriptional suppression of microRNA-27a contributes to laryngeal cancer differentiation via GSK-3β-involved Wnt/β-catenin pathway.Oncotarget. 2017 Feb 28;8(9):14708-14718. doi: 10.18632/oncotarget.14769. Oncotarget. 2017. PMID: 28122350 Free PMC article.
-
MicroRNA-27a Promotes the Proliferation and Invasiveness of Colon Cancer Cells by Targeting SFRP1 through the Wnt/β-Catenin Signaling Pathway.Cell Physiol Biochem. 2017;42(5):1920-1933. doi: 10.1159/000479610. Epub 2017 Aug 3. Cell Physiol Biochem. 2017. Retraction in: Cell Physiol Biochem. 2021;55(1):140. doi: 10.33594/000000349. PMID: 28772260 Retracted.
-
Glycogen synthase kinase 3β in tumorigenesis and oncotherapy (Review).Oncol Rep. 2020 Dec;44(6):2373-2385. doi: 10.3892/or.2020.7817. Epub 2020 Oct 20. Oncol Rep. 2020. PMID: 33125126 Free PMC article. Review.
-
Glycogen Synthase Kinase 3β: A True Foe in Pancreatic Cancer.Int J Mol Sci. 2022 Nov 16;23(22):14133. doi: 10.3390/ijms232214133. Int J Mol Sci. 2022. PMID: 36430630 Free PMC article. Review.
Cited by
-
The protective effect of MiR-27a on the neonatal hypoxic-ischemic encephalopathy by targeting FOXO1 in rats.Transl Pediatr. 2022 Jul;11(7):1199-1208. doi: 10.21037/tp-22-259. Transl Pediatr. 2022. PMID: 35958013 Free PMC article.
-
miR-22-3p/PGC1β Suppresses Breast Cancer Cell Tumorigenesis via PPARγ.PPAR Res. 2021 Mar 12;2021:6661828. doi: 10.1155/2021/6661828. eCollection 2021. PPAR Res. 2021. PMID: 33777130 Free PMC article.
-
Serum-derived extracellular vesicles mediate Smad4 expression through shuttling microRNA-27a in the progression of laryngeal squamous cell carcinoma.Hum Cell. 2022 Jul;35(4):1084-1099. doi: 10.1007/s13577-022-00712-6. Epub 2022 May 12. Hum Cell. 2022. PMID: 35545731
-
Hsa_circ_0002082 up-regulates Centromere Protein F via abolishing miR-508-3p to promote breast cancer progression.J Clin Lab Anal. 2022 Nov;36(11):e24697. doi: 10.1002/jcla.24697. Epub 2022 Sep 26. J Clin Lab Anal. 2022. PMID: 36161346 Free PMC article.
-
miRNAs in the Box: Potential Diagnostic Role for Extracellular Vesicle-Packaged miRNA-27a and miRNA-128 in Breast Cancer.Int J Mol Sci. 2023 Oct 28;24(21):15695. doi: 10.3390/ijms242115695. Int J Mol Sci. 2023. PMID: 37958677 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395–406. - PubMed
-
- Libson S, Lippman M. A review of clinical aspects of breast cancer. Int Rev Psychiatry. 2014;26(1):4–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

