Biofilms deform soft surfaces and disrupt epithelia
- PMID: 33025904
- PMCID: PMC7556879
- DOI: 10.7554/eLife.56533
Biofilms deform soft surfaces and disrupt epithelia
Abstract
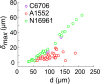
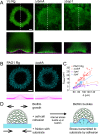
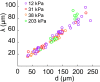
During chronic infections and in microbiota, bacteria predominantly colonize their hosts as multicellular structures called biofilms. A common assumption is that biofilms exclusively interact with their hosts biochemically. However, the contributions of mechanics, while being central to the process of biofilm formation, have been overlooked as a factor influencing host physiology. Specifically, how biofilms form on soft, tissue-like materials remains unknown. Here, we show that biofilms of the pathogens Vibrio cholerae and Pseudomonas aeruginosa can induce large deformations of soft synthetic hydrogels. Biofilms buildup internal mechanical stress as single cells grow within the elastic matrix. By combining mechanical measurements and mutations in matrix components, we found that biofilms deform by buckling, and that adhesion transmits these forces to their substrates. Finally, we demonstrate that V. cholerae biofilms can generate sufficient mechanical stress to deform and even disrupt soft epithelial cell monolayers, suggesting a mechanical mode of infection.
Keywords: Pseudomonas aeruginosa; Vibrio cholerae; biofilm; biomechanics; infectious disease; microbiology.
© 2020, Cont et al.
Conflict of interest statement
AC, TR, ZA, AP No competing interests declared
Figures
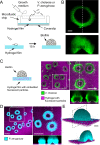
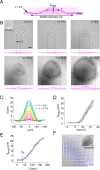
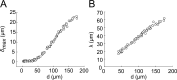
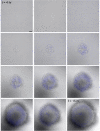



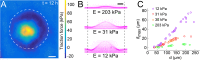



Similar articles
-
Biofilm Matrix Proteins.Microbiol Spectr. 2015 Apr;3(2):10.1128/microbiolspec.MB-0004-2014. doi: 10.1128/microbiolspec.MB-0004-2014. Microbiol Spectr. 2015. PMID: 26104709 Free PMC article. Review.
-
Molecular determinants of mechanical properties of V. cholerae biofilms at the air-liquid interface.Biophys J. 2014 Nov 18;107(10):2245-52. doi: 10.1016/j.bpj.2014.10.015. Biophys J. 2014. PMID: 25418293 Free PMC article.
-
Morphogenesis and cell ordering in confined bacterial biofilms.Proc Natl Acad Sci U S A. 2021 Aug 3;118(31):e2107107118. doi: 10.1073/pnas.2107107118. Proc Natl Acad Sci U S A. 2021. PMID: 34330824 Free PMC article.
-
Living in the matrix: assembly and control of Vibrio cholerae biofilms.Nat Rev Microbiol. 2015 May;13(5):255-68. doi: 10.1038/nrmicro3433. Nat Rev Microbiol. 2015. PMID: 25895940 Free PMC article. Review.
-
Architectural transitions in Vibrio cholerae biofilms at single-cell resolution.Proc Natl Acad Sci U S A. 2016 Apr 5;113(14):E2066-72. doi: 10.1073/pnas.1601702113. Epub 2016 Mar 1. Proc Natl Acad Sci U S A. 2016. PMID: 26933214 Free PMC article.
Cited by
-
Materials science and mechanosensitivity of living matter.Appl Phys Rev. 2022 Mar;9(1):011320. doi: 10.1063/5.0071648. Appl Phys Rev. 2022. PMID: 35392267 Free PMC article. Review.
-
Substrate stiffness impacts early biofilm formation by modulating Pseudomonas aeruginosa twitching motility.Elife. 2023 May 9;12:e81112. doi: 10.7554/eLife.81112. Elife. 2023. PMID: 37158596 Free PMC article.
-
Bacterial lectin BambL acts as a B cell superantigen.Cell Mol Life Sci. 2021 Dec;78(24):8165-8186. doi: 10.1007/s00018-021-04009-z. Epub 2021 Nov 3. Cell Mol Life Sci. 2021. PMID: 34731252 Free PMC article.
-
Vibrio cholerae biofilms use modular adhesins with glycan-targeting and nonspecific surface binding domains for colonization.Nat Commun. 2023 Apr 13;14(1):2104. doi: 10.1038/s41467-023-37660-0. Nat Commun. 2023. PMID: 37055389 Free PMC article.
-
The rat as an animal model in chronic wound research: An update.Life Sci. 2024 Aug 15;351:122783. doi: 10.1016/j.lfs.2024.122783. Epub 2024 Jun 5. Life Sci. 2024. PMID: 38848945 Free PMC article. Review.
References
-
- Bartlett TM, Bratton BP, Duvshani A, Miguel A, Sheng Y, Martin NR, Nguyen JP, Persat A, Desmarais SM, VanNieuwenhze MS, Huang KC, Zhu J, Shaevitz JW, Gitai Z. A periplasmic polymer curves Vibrio cholerae and promotes pathogenesis. Cell. 2017;168:172–185. doi: 10.1016/j.cell.2016.12.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

