RUNX1 marks a luminal castration-resistant lineage established at the onset of prostate development
- PMID: 33025905
- PMCID: PMC7644213
- DOI: 10.7554/eLife.60225
RUNX1 marks a luminal castration-resistant lineage established at the onset of prostate development
Abstract
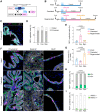
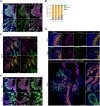
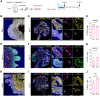
The characterization of prostate epithelial hierarchy and lineage heterogeneity is critical to understand its regenerative properties and malignancies. Here, we report that the transcription factor RUNX1 marks a specific subpopulation of proximal luminal cells (PLCs), enriched in the periurethral region of the developing and adult mouse prostate, and distinct from the previously identified NKX3.1+ luminal castration-resistant cells. Using scRNA-seq profiling and genetic lineage tracing, we show that RUNX1+ PLCs are unaffected by androgen deprivation, and do not contribute to the regeneration of the distal luminal compartments. Furthermore, we demonstrate that a transcriptionally similar RUNX1+ population emerges at the onset of embryonic prostate specification to populate the proximal region of the ducts. Collectively, our results reveal that RUNX1+ PLCs is an intrinsic castration-resistant and self-sustained lineage that emerges early during prostate development and provide new insights into the lineage relationships of the prostate epithelium.
Keywords: RUNX1; development; developmental biology; intrinsic castration-resistance; mouse; prostate; proximal luminal cells; regenerative medicine; single-cell RNA-seq; stem cells.
Plain language summary
The prostate is part of the reproductive organs in male mammals. Many of the cells lining the inside of the prostate – known as ‘luminal cells’ – need hormones to survive. Certain treatments for prostate cancer, including surgical and chemical castration, lead to fewer hormones reaching the prostate, which shrinks as luminal cells die. But some of these luminal cells are able to survive the damaging effects of castration, rebuilding the prostate upon treatment with hormones, which can lead to the cancer reappearing. It is unclear which type of luminal cells survive during periods without hormones and are responsible for regenerating the prostate. RUNX1 is a protein responsible for switching genes on and off, and is usually found in blood cells, which it helps to mature and perform their roles, but has also been detected in tissues that depend on hormones. Since the luminal cells of the prostate rely on hormones, could RUNX1 also be present in these cells? To answer this question, Mével et al. used mice to determine where and when RUNX1 is found in prostate cells. Mével et al. detected high levels of RUNX1 in a patch of luminal cells at the base of the prostate. Samples of these cells were taken for further testing from developing mouse embryos, healthy adult mice and mice in which the prostate was regenerating after surgical castration. Mével et al. found that these cells were a distinct subtype of luminal cells that were able to resist the effects of castration – they survived without hormones. Though these cells were present during the early stages of prostate embryonic development and in healthy adult prostate tissue, they were not responsible for rebuilding the prostate after castration. Mével et al.’s results indicate that, in mice, RUNX1 may act as a marker for a subset of luminal cells that can survive after castration. Further probing the roles of these castration-resistant luminal cells in normal and cancerous prostate tissue may improve the outcome of patients with prostate cancer treated with hormone deprivation therapy.
© 2020, Mevel et al.
Conflict of interest statement
RM, IS, SM, LG, RP, MF, IA, HL, PO, KB, EB, GL No competing interests declared
Figures


















Similar articles
-
Bmi1 marks distinct castration-resistant luminal progenitor cells competent for prostate regeneration and tumour initiation.Nat Commun. 2016 Oct 5;7:12943. doi: 10.1038/ncomms12943. Nat Commun. 2016. PMID: 27703144 Free PMC article.
-
Urethral luminal epithelia are castration-insensitive cells of the proximal prostate.Prostate. 2020 Aug;80(11):872-884. doi: 10.1002/pros.24020. Epub 2020 Jun 4. Prostate. 2020. PMID: 32497356 Free PMC article.
-
A Runx1-enhancer Element eR1 Identified Lineage Restricted Mammary Luminal Stem Cells.Stem Cells. 2022 Mar 3;40(1):112-122. doi: 10.1093/stmcls/sxab009. Stem Cells. 2022. PMID: 35511868
-
Heterogeneity and complexity of the prostate epithelium: New findings from single-cell RNA sequencing studies.Cancer Lett. 2022 Jan 28;525:108-114. doi: 10.1016/j.canlet.2021.10.035. Epub 2021 Oct 30. Cancer Lett. 2022. PMID: 34728312 Free PMC article. Review.
-
Prostate luminal progenitor cells: from mouse to human, from health to disease.Nat Rev Urol. 2022 Apr;19(4):201-218. doi: 10.1038/s41585-021-00561-2. Epub 2022 Jan 25. Nat Rev Urol. 2022. PMID: 35079142 Review.
Cited by
-
ESS2 controls prostate cancer progression through recruitment of chromodomain helicase DNA binding protein 1.Sci Rep. 2023 Jul 31;13(1):12355. doi: 10.1038/s41598-023-39626-0. Sci Rep. 2023. PMID: 37524814 Free PMC article.
-
Prostate cancer cell heterogeneity and plasticity: Insights from studies of genetically-engineered mouse models.Semin Cancer Biol. 2022 Jul;82:60-67. doi: 10.1016/j.semcancer.2021.06.016. Epub 2021 Jun 18. Semin Cancer Biol. 2022. PMID: 34147640 Free PMC article. Review.
-
Progenitors in prostate development and disease.Dev Biol. 2021 May;473:50-58. doi: 10.1016/j.ydbio.2020.11.012. Epub 2021 Jan 30. Dev Biol. 2021. PMID: 33529704 Free PMC article. Review.
-
Integrated single-cell analysis defines the epigenetic basis of castration-resistant prostate luminal cells.Cell Stem Cell. 2024 Aug 1;31(8):1203-1221.e7. doi: 10.1016/j.stem.2024.05.008. Epub 2024 Jun 14. Cell Stem Cell. 2024. PMID: 38878775 Free PMC article.
-
Prostate organogenesis.Development. 2022 Jun 15;149(12):dev200394. doi: 10.1242/dev.200394. Epub 2022 Jun 21. Development. 2022. PMID: 35726824 Free PMC article.
References
-
- Aibar S, González-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, Aerts S. SCENIC: single-cell regulatory network inference and clustering. Nature Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. - DOI - PMC - PubMed
-
- Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Scientific Reports. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. - DOI - PMC - PubMed
-
- Barros-Silva JD, Linn DE, Steiner I, Guo G, Ali A, Pakula H, Ashton G, Peset I, Brown M, Clarke NW, Bronson RT, Yuan GC, Orkin SH, Li Z, Baena E. Single-Cell analysis identifies LY6D as a marker linking Castration-Resistant prostate luminal cells to prostate progenitors and Cancer. Cell Reports. 2018;25:3504–3518. doi: 10.1016/j.celrep.2018.11.069. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

