Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001
- PMID: 33026184
- PMCID: PMC8369809
- DOI: 10.1002/pbc.28719
Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001
Erratum in
-
Corrigendum.Pediatr Blood Cancer. 2021 Mar;68(3):e28885. doi: 10.1002/pbc.28885. Epub 2020 Dec 31. Pediatr Blood Cancer. 2021. PMID: 33506554 No abstract available.
Abstract
Background/objectives: While outcomes for pediatric T-cell acute lymphoblastic leukemia (T-ALL) are favorable, there are few widely accepted prognostic factors, limiting the ability to risk stratify therapy.
Design/methods: Dana-Farber Cancer Institute (DFCI) Protocols 05-001 and 11-001 enrolled pediatric patients with newly diagnosed B- or T-ALL from 2005 to 2011 and from 2012 to 2015, respectively. Protocol therapy was nearly identical for patients with T-ALL (N = 123), who were all initially assigned to the high-risk arm. End-induction minimal residual disease (MRD) was assessed by reverse transcription polymerase chain reaction (RT-PCR) or next-generation sequencing (NGS), but was not used to modify postinduction therapy. Early T-cell precursor (ETP) status was determined by flow cytometry. Cases with sufficient diagnostic DNA were retrospectively evaluated by targeted NGS of known genetic drivers of T-ALL, including Notch, PI3K, and Ras pathway genes.
Results: The 5-year event-free survival (EFS) and overall survival (OS) for patients with T-ALL was 81% (95% CI, 73-87%) and 90% (95% CI, 83-94%), respectively. ETP phenotype was associated with failure to achieve complete remission, but not with inferior OS. Low end-induction MRD (<10-4 ) was associated with superior disease-free survival (DFS). Pathogenic mutations of the PI3K pathway were mutually exclusive of ETP phenotype and were associated with inferior 5-year DFS and OS.
Conclusions: Together, our findings demonstrate that ETP phenotype, end-induction MRD, and PI3K pathway mutation status are prognostically relevant in pediatric T-ALL and should be considered for risk classification in future trials. DFCI Protocols 05-001 and 11-001 are registered at www.clinicaltrials.gov as NCT00165087 and NCT01574274, respectively.
Keywords: ALL; T-ALL; clinical trials; minimal residual disease; molecular diagnosis and therapy; pediatric oncology.
© 2020 The Authors. Pediatric Blood & Cancer published by Wiley Periodicals, Inc.
Conflict of interest statement
Figures

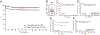

References
-
- Goldberg JM, Silverman LB, Levy DE, et al.Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(19):3616–3622. - PubMed
-
- Hunger SP, Lu X, Devidas M, et al.Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(14):1663–1669. - PMC - PubMed
-
- Smith M, Arthur D, Camitta B, et al.Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(1):18–24. - PubMed
-
- Conter V, Bartram CR, Valsecchi MG, et al.Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

