Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial
- PMID: 33026243
- PMCID: PMC7717477
- DOI: 10.1161/CIRCULATIONAHA.120.050255
Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial
Abstract
Background: In patients with type 2 diabetes mellitus, sodium-glucose cotransporter 2 inhibitors reduce the risk of hospitalization for heart failure (HHF). We assessed the effect of ertugliflozin on HHF and related outcomes.
Methods: VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial), a double-blind, placebo-controlled trial, randomly assigned patients with type 2 diabetes mellitus and atherosclerotic cardiovascular (CV) disease to once-daily ertugliflozin 5 mg, 15 mg, or placebo. Prespecified secondary analyses compared ertugliflozin (pooled doses) versus placebo on time to first event of HHF and composite of HHF/CV death, overall and stratified by prespecified characteristics. Cox proportional hazards modeling was used with the Fine and Gray method to account for competing mortality risk, and Andersen-Gill modeling to analyze total (first+recurrent) HHF and total HHF/CV death events.
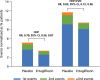
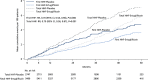
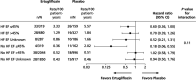
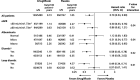
Results: A total of 8246 patients were randomly assigned to ertugliflozin (n=5499) or placebo (n=2747); n=1958 (23.7%) had a history of heart failure (HF) and n=5006 (60.7%) had pretrial ejection fraction (EF) available, including n=959 with EF ≤45%. Ertugliflozin did not significantly reduce first HHF/CV death (hazard ratio [HR], 0.88 [95% CI, 0.75-1.03]). Overall, ertugliflozin reduced risk for first HHF (HR, 0.70 [95% CI, 0.54-0.90]; P=0.006). Previous HF did not modify this effect (HF: HR, 0.63 [95% CI, 0.44-0.90]; no HF: HR, 0.79 [95% CI, 0.54-1.15]; P interaction=0.40). In patients with HF, the risk reduction for first HHF was similar for those with reduced EF ≤45% versus preserved EF >45% or unknown. However, in the overall population, the risk reduction tended to be greater for those with EF ≤45% (HR, 0.48 [95% CI, 0.30-0.76]) versus EF >45% (HR, 0.86 [95% CI, 0.58-1.29]). Effect on risk for first HHF was consistent across most subgroups, but greater benefit of ertugliflozin was observed in 3 populations: baseline estimated glomerular filtration rate <60 mL·min-1·1.73 m-2, albuminuria, and diuretic use (each P interaction <0.05). Ertugliflozin reduced total events of HHF (rate ratio, 0.70 [95% CI, 0.56-0.87]) and total HHF/CV death (rate ratio, 0.83 [95% CI, 0.72-0.96]).
Conclusions: In patients with type 2 diabetes mellitus, ertugliflozin reduced the risk for first and total HHF and total HHF/CV death, adding further support for the use of sodium-glucose cotransporter 2 inhibitors in primary and secondary prevention of HHF. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01986881.
Keywords: cardiovascular diseases; diabetes mellitus, type 2; heart failure; sodium-glucose cotransporter 2 inhibitors.
Conflict of interest statement
Dr Cosentino has received research grants from Swedish Research Council, Swedish Heart & Lung Foundation, and King Gustav V and Queen Victoria Foundation, and fees from Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme Corp, Mundipharma, Novo Nordisk, and Pfizer, as well. Dr Cannon has received research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Merck & Co, Inc, Pfizer, and fees from Aegerion, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol-Myers Squibb, Corvidia, Eli Lilly, HLS Therapeutics, Innovent, Janssen, Kowa, Merck & Co, Inc, Pfizer, Rhoshan, and Sanofi, as well. Dr Cherney has received consulting fees or speaking honoraria or both from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co, Inc, Mitsubishi-Tanabe, Novo Nordisk, Prometic, and Sanofi, and has received operating funds from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co, Inc, and Sanofi. Dr Pratley has received fees (directed to his institution) from AstraZeneca, Glytec, LLC, Hanmi Pharmaceutical Co Ltd, Janssen, Lexicon Pharmaceuticals, Inc, Merck & Co, Inc, Mundipharma, Novo Nordisk, Pfizer, Poxel SA, Sanofi, Sanofi US Services, Inc, Scohia Pharma Inc, and Sun Pharmaceutical Industries. Dr Dagogo-Jack has led clinical trials for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, Inc; has received consulting fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck & Co, Inc, and Sanofi; and has equity interests in Jana Care, Inc and Aerami Therapeutics. Dr Charbonnel has received fees from AstraZeneca, Boehringer Ingelheim, Lilly, Merck Sharpe & Dohme, Mundipharma, Novo Nordisk, Sanofi, and Takeda. Dr McGuire has received honoraria or consultancy fees from Afimmune, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eisai, Esperion, GlaxoSmithKline, Janssen Research and Development LLC, Lexicon, Lilly USA, Merck & Co, Inc, Metavant, Novo Nordisk, Pfizer, and Sanofi US. Dr Gantz is an employee of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, who owns stock in the company. Drs Frederich, Mancuso, Terra, Cater, and Masiukiewicz are employees and shareholders of Pfizer Inc. Dr Shih reports no conflicts.
Figures


Comment in
-
VERTIS-CV: More Evidence That Sodium Glucose Cotransporter 2 Inhibition Brings Rapid and Sustained Heart Failure Benefit.Circulation. 2020 Dec 8;142(23):2216-2218. doi: 10.1161/CIRCULATIONAHA.120.050512. Epub 2020 Dec 7. Circulation. 2020. PMID: 33284652 No abstract available.
References
-
- McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. doi: 10.1016/S2213-8587(14)70031-2 - PubMed
-
- Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256 - PubMed
-
- Butler J, Januzzi JL, Rosenstock J. Management of heart failure and type 2 diabetes mellitus: maximizing complementary drug therapy. Diabetes Obes Metab. 2020;22:1243–1262. doi: 10.1111/dom.14042 - PubMed
-
- McGuire DK, Van de Werf F, Armstrong PW, Standl E, Koglin J, Green JB, Bethel MA, Cornel JH, Lopes RD, Halvorsen S, et al. ; Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–135. doi: 10.1001/jamacardio.2016.0103 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

