Lack of airway submucosal glands impairs respiratory host defenses
- PMID: 33026343
- PMCID: PMC7541087
- DOI: 10.7554/eLife.59653
Lack of airway submucosal glands impairs respiratory host defenses
Abstract
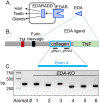
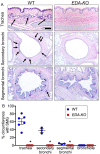
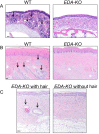
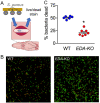
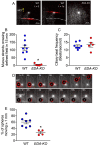
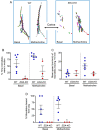
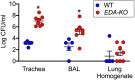
Submucosal glands (SMGs) are a prominent structure that lines human cartilaginous airways. Although it has been assumed that SMGs contribute to respiratory defense, that hypothesis has gone without a direct test. Therefore, we studied pigs, which have lungs like humans, and disrupted the gene for ectodysplasin (EDA-KO), which initiates SMG development. EDA-KO pigs lacked SMGs throughout the airways. Their airway surface liquid had a reduced ability to kill bacteria, consistent with SMG production of antimicrobials. In wild-type pigs, SMGs secrete mucus that emerges onto the airway surface as strands. Lack of SMGs and mucus strands disrupted mucociliary transport in EDA-KO pigs. Consequently, EDA-KO pigs failed to eradicate a bacterial challenge in lung regions normally populated by SMGs. These in vivo and ex vivo results indicate that SMGs are required for normal antimicrobial activity and mucociliary transport, two key host defenses that protect the lung.
Keywords: host defense; lung; medicine; sus scrofa.
© 2020, Ostedgaard et al.
Conflict of interest statement
LO, MP, KW, MA, AF, AW, MS, LS, PA, BH, GR, MO, BG, SM, LP, MS, NG, CH, KZ, JG, TB, EH, DM, RP, DS, MW No competing interests declared
Figures



References
-
- Ballard ST, Fountain JD, Inglis SK, Corboz MR, Taylor AE. Chloride secretion across distal airway epithelium: relationship to submucosal gland distribution. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1995;268:L526–L531. doi: 10.1152/ajplung.1995.268.3.L526. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL135433/HL/NHLBI NIH HHS/United States
- S10 OD018526/OD/NIH HHS/United States
- T32 AI007533/AI/NIAID NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- P01 HL091842/HL/NHLBI NIH HHS/United States
- R01 HL136813/HL/NHLBI NIH HHS/United States
- CFF FISCHE16I0/Cystic Fibrosis Foundation/International
- P01 HL051670/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- P01 HL152960/HL/NHLBI NIH HHS/United States
- T32 HL007638/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CFF STOLTZ16XX0/Cystic Fibrosis Foundation/International
- K08 HL136927/HL/NHLBI NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

