Multimodal imaging reveals a unique autofluorescence signature of Randall's plaque
- PMID: 33026465
- PMCID: PMC7965236
- DOI: 10.1007/s00240-020-01216-4
Multimodal imaging reveals a unique autofluorescence signature of Randall's plaque
Abstract
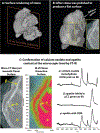
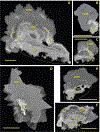
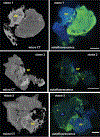
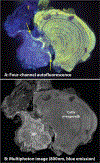
Kidney stones frequently develop as an overgrowth on Randall's plaque (RP) which is formed in the papillary interstitium. The organic composition of RP is distinct from stone matrix in that RP contains fibrillar collagen; RP in tissue has also been shown to have two proteins that are also found in stones, but otherwise the molecular constituents of RP are unstudied. We hypothesized that RP contains unique organic molecules that can be differentiated from the stone overgrowth by fluorescence. To test this, we used micro-CT-guided polishing to expose the interior of kidney stones for multimodal imaging with multiphoton, confocal and infrared microscopy. We detected a blue autofluorescence signature unique to RP, the specificity of which was also confirmed in papillary tissue from patients with stone disease. High-resolution mineral mapping of the stone also showed a transition from the apatite within RP to the calcium oxalate in the overgrowth, demonstrating the molecular and spatial transition from the tissue to the urine. This work provides a systematic and practical approach to uncover specific fluorescence signatures which correlate with mineral type, verifies previous observations regarding mineral overgrowth onto RP and identifies a novel autofluorescence signature of RP demonstrating RP's unique molecular composition.
Keywords: Calcium oxalate; Fluorescence microscopy; Infrared spectroscopy; Kidney stones; Micro-CT; Nephrolithiasis.
Conflict of interest statement
Conflicts of interest: None
Figures



References
-
- Verrier C, Bazin D, Huguet L, Stéphan O, Gloter A, Verpont M-C, Frochot V, Haymann J-P, Brocheriou I, Traxer O, Daudon M, Letavernier E (2016) Topography, composition and structure of incipient Randall’s plaque at the nanoscale level. J Urol 196: 1566–1574 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

