The Impact of OnabotulinumtoxinA vs. Placebo on Efficacy Outcomes in Headache Day Responder and Nonresponder Patients with Chronic Migraine
- PMID: 33026630
- PMCID: PMC7648816
- DOI: 10.1007/s40122-020-00199-9
The Impact of OnabotulinumtoxinA vs. Placebo on Efficacy Outcomes in Headache Day Responder and Nonresponder Patients with Chronic Migraine
Abstract
Introduction: The phase 3 PREEMPT trials demonstrated efficacy and tolerability of onabotulinumtoxinA for headache prevention in adults with chronic migraine. OnabotulinumtoxinA significantly reduced headache frequency from baseline vs. placebo at 24 weeks; however, this measure may not fully capture the benefits of treatment. We evaluated the impact of onabotulinumtoxinA on patient-reported outcomes according to headache responder status.
Methods: A post hoc analysis pooled 24-week data from the placebo-controlled, randomized, double-blind treatment phases of the PREEMPT trials. Patients were stratified by randomized treatment (onabotulinumtoxinA vs. placebo) and headache day responder status (responder vs. nonresponder). Headache day responders had a ≥ 50% headache day reduction from baseline measured at weeks 21-24. Outcomes evaluated were patient-reported reductions in moderate-to-severe headache days, Headache Impact Test, and Migraine-Specific Quality of Life Questionnaire. Missing values were estimated using a modified last-observation-carried-forward approach.
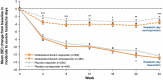
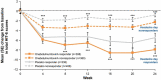
Results: In the pooled analysis population (N = 1384; onabotulinumtoxinA, n = 688; placebo, n = 696), headache day responder rates were 308/688 (45%) for onabotulinumtoxinA- and 238/696 (34%) for placebo-treated patients. At 24 weeks compared with baseline, onabotulinumtoxinA nonresponders showed significantly (all P < 0.01) greater mean (standard error) reductions vs. placebo nonresponders in moderate-to-severe headache days (- 3.5 [0.2] vs. - 2.4 [0.2]) and Headache Impact Test scores (- 2.3 [0.3] vs. - 0.8 [0.2]), and greater mean improvements in Migraine-Specific Quality of Life Questionnaire domains (Restrictive, 8.8 [1.0] vs. 2.9 [0.8]; Preventive, 6.0 [1.0] vs. 1.8 [0.8]; Emotional, 8.5 [1.3] vs. 2.8 [1.1]). Moderate-to-severe headache day and headache impact differences between nonresponder groups were evident at week 4 and sustained through week 24.
Conclusions: Relative to placebo nonresponders, onabotulinumtoxinA nonresponders experienced significant reductions in moderate-to-severe headache days and disability and improvement in quality of life, implying that the full benefits of onabotulinumtoxinA are not captured by headache day reduction.
Trial registration: ClinicalTrials.gov identifiers, NCT00156910 (PREEMPT 1) and NCT00168428 (PREEMPT 2).
Keywords: Botulinum toxin type A; Chronic migraine; Headache; Quality of life.
Figures

References
-
- Headache Classification Committee of the International Headache Society The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. - PubMed
-
- Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A Comparison of the chronic migraine epidemiology and outcomes (CaMEO) study and American migraine prevalence and prevention (AMPP) Study: demographics and headache-related disability. Headache. 2016;56:1280–1289. doi: 10.1111/head.12878. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

