Continuous Flow Sodiation of Substituted Acrylonitriles, Alkenyl Sulfides and Acrylates
- PMID: 33026681
- PMCID: PMC7821005
- DOI: 10.1002/anie.202012085
Continuous Flow Sodiation of Substituted Acrylonitriles, Alkenyl Sulfides and Acrylates
Abstract
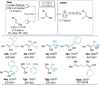
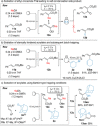
The sodiation of substituted acrylonitriles and alkenyl sulfides in a continuous flow set-up using NaDA (sodium diisopropylamide) in EtNMe2 or NaTMP (sodium 2,2,6,6-tetramethylpiperidide)⋅TMEDA in n-hexane provides sodiated acrylonitriles and alkenyl sulfides, which are subsequently trapped in batch with various electrophiles such as aldehydes, ketones, disulfides and allylic bromides affording functionalized acrylonitriles and alkenyl sulfides. This flow-procedure was successfully extended to other acrylates by using Barbier-type conditions.
Keywords: Barbier-type reactions; acrylonitriles; alkenyl sulfides; flow chemistry; sodiation.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- None
-
- Fleming F. F., Wang Q., Zhang Z., Steward O. W., J. Org. Chem. 2002, 67, 5953; - PubMed
-
- Doroszuk J., Musiejuk M., Ponikiewski L., Witt D., Eur. J. Org. Chem. 2018, 6333;
-
- De Lucchi O., Pasquato L., Tetrahedron 1988, 44, 6755;
-
- Trost B. M., Lavoie A. C., J. Am. Chem. Soc. 1983, 105, 5075;
Publication types
LinkOut - more resources
Full Text Sources

