Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain
- PMID: 33027637
- PMCID: PMC7642038
- DOI: 10.1016/j.cmet.2020.09.011
Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain
Abstract
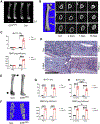
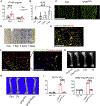
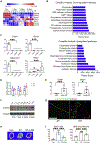
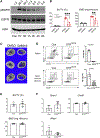
Adipocytes control bone mass, but the mechanism is unclear. To explore the effect of postnatal adipocyte elimination on bone cells, we mated mice expressing an inducible primate diphtheria toxin receptor (DTR) to those bearing adiponectin (ADQ)-Cre. DTR activation eliminates peripheral and marrow adipocytes in these DTRADQ mice. Within 4 days of DTR activation, the systemic bone mass of DTRADQ mice began to increase due to stimulated osteogenesis, with a 1,000% expansion by 10-14 days post-DTR treatment. This adipocyte ablation-mediated enhancement of skeletal mass reflected bone morphogenetic protein (BMP) receptor activation following the elimination of its inhibitors, associated with simultaneous epidermal growth factor (EGF) receptor signaling. DTRADQ-induced osteosclerosis is not due to ablation of peripheral adipocytes but likely reflects the elimination of marrow ADQ-expressing cells. Thus, anabolic drugs targeting BMP receptor inhibitors with short-term EGF receptor activation may be a means of profoundly increasing skeletal mass to prevent or reverse pathological bone loss.
Keywords: BMPR activation; adipocyte; bone formation; heparin-binding epidermal like growth factor.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests R.A.B. may receive royalty income based on the CompBio technology developed by R.A.B. and licensed by Washington University to PercayAI. All other authors declare no competing interests.
Figures



References
-
- Ahn J, Serrano de la Pena L, Shore EM, and Kaplan FS (2003). Paresis of a bone morphogenetic protein-antagonist response in a genetic disorder of heterotopic skeletogenesis. J. Bone Joint Surg. Am 85-a, 667–674. - PubMed
-
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ, and Pa THSI (2005). One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N. Engl. J. Med 353, 555–565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

