The Anaerobic Product Ethanol Promotes Autophagy-Dependent Submergence Tolerance in Arabidopsis
- PMID: 33028029
- PMCID: PMC7583018
- DOI: 10.3390/ijms21197361
The Anaerobic Product Ethanol Promotes Autophagy-Dependent Submergence Tolerance in Arabidopsis
Abstract
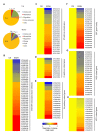
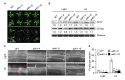
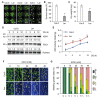
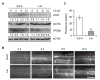
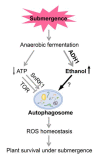
In response to hypoxia under submergence, plants switch from aerobic respiration to anaerobic fermentation, which leads to the accumulation of the end product, ethanol. We previously reported that Arabidopsis thaliana autophagy-deficient mutants show increased sensitivity to ethanol treatment, indicating that ethanol is likely involved in regulating the autophagy-mediated hypoxia response. Here, using a transcriptomic analysis, we identified 3909 genes in Arabidopsis seedlings that were differentially expressed in response to ethanol treatment, including 2487 upregulated and 1422 downregulated genes. Ethanol treatment significantly upregulated genes involved in autophagy and the detoxification of reactive oxygen species. Using transgenic lines expressing AUTOPHAGY-RELATED PROTEIN 8e fused to green fluorescent protein (GFP-ATG8e), we confirmed that exogenous ethanol treatment promotes autophagosome formation in vivo. Phenotypic analysis showed that deletions in the alcohol dehydrogenase gene in adh1 mutants result in attenuated submergence tolerance, decreased accumulation of ATG proteins, and diminished submergence-induced autophagosome formation. Compared to the submergence-tolerant Arabidopsis accession Columbia (Col-0), the submergence-intolerant accession Landsberg erecta (Ler) displayed hypersensitivity to ethanol treatment; we linked these phenotypes to differences in the functions of ADH1 and the autophagy machinery between these accessions. Thus, ethanol promotes autophagy-mediated submergence tolerance in Arabidopsis.
Keywords: ADH1; autophagy; ethanol; hypoxia; submergence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sasidharan R., Bailey-Serres J., Ashikari M., Atwell B.J., Colmer T.D., Fagerstedt K., Fukao T., Geigenberger P., Hebelstrup K.H., Hill R.D., et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017;214:1403–1407. doi: 10.1111/nph.14519. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

