Oxidative Stress Product, 4-Hydroxy-2-Nonenal, Induces the Release of Tissue Factor-Positive Microvesicles From Perivascular Cells Into Circulation
- PMID: 33028097
- PMCID: PMC7752210
- DOI: 10.1161/ATVBAHA.120.315187
Oxidative Stress Product, 4-Hydroxy-2-Nonenal, Induces the Release of Tissue Factor-Positive Microvesicles From Perivascular Cells Into Circulation
Abstract
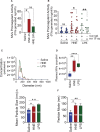
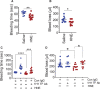
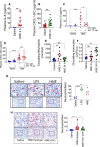
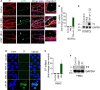
Objective: TF (Tissue factor) plays a key role in hemostasis, but an aberrant expression of TF leads to thrombosis. The objective of the present study is to investigate the effect of 4-hydroxy-2-nonenal (HNE), the most stable and major oxidant produced in various disease conditions, on the release of TF+ microvesicles into the circulation, identify the source of TF+ microvesicles origin, and assess their effect on intravascular coagulation and inflammation. Approach and Results: C57BL/6J mice were administered with HNE intraperitoneally, and the release of TF+ microvesicles into circulation was evaluated using coagulation assays and nanoparticle tracking analysis. Various cell-specific markers were used to identify the cellular source of TF+ microvesicles. Vascular permeability was analyzed by the extravasation of Evans blue dye or fluorescein dextran. HNE administration to mice markedly increased the levels of TF+ microvesicles and thrombin generation in the circulation. HNE administration also increased the number of neutrophils in the lungs and elevated the levels of inflammatory cytokines in plasma. Administration of an anti-TF antibody blocked not only HNE-induced thrombin generation but also HNE-induced inflammation. Confocal microscopy and immunoblotting studies showed that HNE does not induce TF expression either in vascular endothelium or circulating monocytes. Microvesicles harvested from HNE-administered mice stained positively with CD248 and α-smooth muscle actin, the markers that are specific to perivascular cells. HNE was found to destabilize endothelial cell barrier integrity.
Conclusions: HNE promotes the release of TF+ microvesicles from perivascular cells into the circulation. HNE-induced increased TF activity contributes to intravascular coagulation and inflammation.
Keywords: HNE; hemostasis; inflammation; neutrophils; oxidative stress; thrombosis; tissue factor.
Conflict of interest statement
None.
Figures


Comment in
-
New Cellular Source of TF (Tissue Factor)-Positive Extracellular Vesicles in the Circulation.Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):266-268. doi: 10.1161/ATVBAHA.120.315437. Epub 2020 Dec 23. Arterioscler Thromb Vasc Biol. 2021. PMID: 33356372 Free PMC article. No abstract available.
References
-
- Rapaport SI, Rao LV. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost. 1995;74:7–17 - PubMed
-
- Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846 - PubMed
-
- Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6 - PubMed
-
- Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

