Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study
- PMID: 33028559
- PMCID: PMC7539604
- DOI: 10.1136/bmjopen-2020-038276
Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study
Abstract
Introduction: Although topical use of moisturisers is slightly effective for the prevention and avoiding the aggravation of hand-foot syndrome induced by multikinase inhibitors, there is still room for improvement. Hydrocolloid dressing is a type of wound dressing often used for wounds such as decubitus ulcers. The purpose of this study is to verify the usefulness of application of hydrocolloid dressings as prophylaxis against development of hand-foot syndrome induced by multikinase inhibitors by comparing the effects of this dressing and standard supportive care (moisturising care alone) within the same individuals.
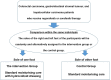
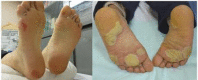
Methods: This study is a phase 3 randomised, self-controlled study to compare prophylactic moisturising care with or without hydrocolloid dressing for hand-foot syndrome induced by multikinase inhibitors. Patients with radically unresectable advanced or recurrent colorectal carcinoma, gastrointestinal stromal tumour and hepatocellular carcinoma who scheduled to receive regorafenib or sorafenib therapy are eligible for enrolment.Supportive care for hand-foot syndrome will consist of basic moisturising care with or without hydrocolloid dressing. If hand-foot syndrome occurs, a steroid ointment will be applied two times per day at the affected sites. The primary endpoint is an incidence rate of grade 2 or more severe hand-foot syndrome (soles of the feet only) assessed by National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events V.4.0. Grading of hand-foot syndrome will be performed by central review using photographs taken weekly by blinded trained physicians. The ethical approval was obtained from National Cancer Center Hospital. The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conference.
Discussion: If the positive results are found in this study, it is shown that hydrocolloid dressing is effective not only as a symptom management but also as a prevention in hand-foot syndrome induced by multikinase.
Trial status: The enrolment was started in January 2019, and planned to closed in January 2021. As of February 2020, 26 patients enrolled in this study.
Trial registration number: UMIN Clinical Trial Registry (UMIN000034853).
Protocol version: V.1.4, 9 January 2020.
Keywords: hand-foot syndrome; multi-kinase inhibitor; self-controlled study.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Abrams TJ, Lee LB, Murray LJ, et al. . Su11248 inhibits kit and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003;2:471–8. - PubMed
-
- Mendel DB, Laird AD, Xin X, et al. . In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
