The HK5 and HK6 cytokinin receptors mediate diverse developmental pathways in rice
- PMID: 33028608
- PMCID: PMC7648598
- DOI: 10.1242/dev.191734
The HK5 and HK6 cytokinin receptors mediate diverse developmental pathways in rice
Erratum in
-
Correction: The HK5 and HK6 cytokinin receptors mediate diverse developmental pathways in rice.Development. 2023 Aug 1;150(15):dev202221. doi: 10.1242/dev.202221. Epub 2023 Aug 1. Development. 2023. PMID: 37526652 Free PMC article. No abstract available.
Abstract
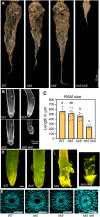
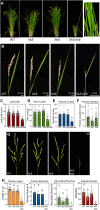
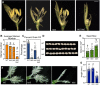
The phytohormone cytokinin regulates diverse aspects of plant growth and development. Our understanding of the metabolism and perception of cytokinin has made great strides in recent years, mostly from studies of the model dicot Arabidopsis Here, we employed a CRISPR/Cas9-based approach to disrupt a subset of cytokinin histidine kinase (HK) receptors in rice (Oryza sativa) in order to explore the role of cytokinin in a monocot species. In hk5 and hk6 single mutants, the root growth, leaf width, inflorescence architecture and/or floral development were affected. The double hk5 hk6 mutant showed more substantial defects, including severely reduced root and shoot growth, a smaller shoot apical meristem, and an enlarged root cap. Flowering was delayed in the hk5 hk6 mutant and the panicle was significantly reduced in size and infertile due to multiple defects in floral development. The hk5 hk6 mutant also exhibited a severely reduced cytokinin response, consistent with the developmental phenotypes arising from a defect in cytokinin signaling. These results indicate that HK5 and HK6 act as cytokinin receptors, with overlapping functions to regulate diverse aspects of rice growth and development.
Keywords: Cell signaling; Cytokinin; Plant development; Plant hormones.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Abramoff M. D., Magelhaes P. J. and Ram S. J. (2004). Image processing with ImageJ. Biophoton. Int. 11, 36-42.
-
- Argyros R. D., Mathews D. E., Chiang Y.-H., Palmer C. M., Thibault D. M., Etheridge N., Argyros D. A., Mason M. G., Kieber J. J. and Schaller G. E. (2008). Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20, 2102-2116. 10.1105/tpc.108.059584 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

