Advances in targeted therapy for esophageal cancer
- PMID: 33028804
- PMCID: PMC7542465
- DOI: 10.1038/s41392-020-00323-3
Advances in targeted therapy for esophageal cancer
Abstract
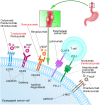
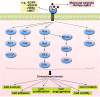
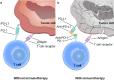
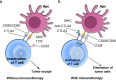
Esophageal cancer (EC) is one of the most lethal cancers in the world, and its morbidity and mortality rates rank among the top ten in China. Currently, surgical resection, radiotherapy and chemotherapy are the primary clinical treatments for esophageal cancer. However, outcomes are still unsatisfactory due to the limited efficacy and severe adverse effects of conventional treatments. As a new type of approach, targeted therapies have been confirmed to play an important role in the treatment of esophageal cancer; these include cetuximab and bevacizumab, which target epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF), respectively. In addition, other drugs targeting surface antigens and signaling pathways or acting on immune checkpoints have been continuously developed. For example, trastuzumab, a monoclonal antibody targeting human epidermal growth factor receptor 2 (HER-2), has been approved by the Food and Drug Administration (FDA) as a first-line treatment of HER-2-positive cancer. Moreover, the PD-L1 inhibitor pembrolizumab has been approved as a highly efficient drug for patients with PD-L1-positive or advanced esophageal squamous cell carcinoma (ESCC). These novel drugs can be used alone or in combination with other treatment strategies to further improve the treatment efficacy and prognosis of cancer patients. Nevertheless, adverse events, optimal dosages and effective combinations still need further investigation. In this review, we expound an outline of the latest advances in targeted therapies of esophageal cancer and the mechanisms of relevant drugs, discuss their efficacy and safety, and provide a clinical rationale for precision medicine in esophageal cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81773085/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81973339/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81803551/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81672953/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31770888/National Natural Science Foundation of China (National Science Foundation of China)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

