In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects
- PMID: 33028837
- PMCID: PMC7542443
- DOI: 10.1038/s41598-020-72978-5
In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects
Abstract
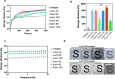
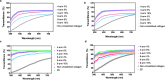
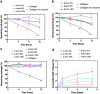
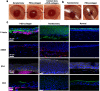
Visually significant corneal injuries and subsequent scarring collectively represent a major global human health challenge, affecting millions of people worldwide. Unfortunately, less than 2% of patients who could benefit from a sight-restoring corneal transplant have access to cadaveric donor corneal tissue. Thus, there is a critical need for new ways to repair corneal defects that drive proper epithelialization and stromal remodeling of the wounded area without the need for cadeveric donor corneas. Emerging therapies to replace the need for donor corneas include pre-formed biosynthetic buttons and in situ-forming matrices that strive to achieve the transparency, biocompatibility, patient comfort, and biointegration that is possible with native tissue. Herein, we report on the development of an in situ-forming hydrogel of collagen type I crosslinked via multi-functional polyethylene glycol (PEG)-N-hydroxysuccinimide (NHS) and characterize its biophysical properties and regenerative capacity both in vitro and in vivo. The hydrogels form under ambient conditions within minutes upon mixing without the need for an external catalyst or trigger such as light or heat, and their transparency, degradability, and stiffness are modulated as a function of number of PEG arms and concentration of PEG. In addition, in situ-forming PEG-collagen hydrogels support the migration and proliferation of corneal epithelial and stromal cells on their surface. In vivo studies in which the hydrogels were formed in situ over stromal keratectomy wounds without sutures showed that they supported multi-layered surface epithelialization. Overall, the in situ forming PEG-collagen hydrogels exhibited physical and biological properties desirable for a corneal stromal defect wound repair matrix that could be applied without the need for sutures or an external trigger such as a catalyst or light energy.
Conflict of interest statement
Author D.M. holds a patent on the portable ophthalmic camera system used to photograph the animal eyes, and authors D.M., GM.F-C, and H.L. have a patent application on the hydrogel technology used in the studies. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

