Microglia-organized scar-free spinal cord repair in neonatal mice
- PMID: 33029008
- PMCID: PMC7704837
- DOI: 10.1038/s41586-020-2795-6
Microglia-organized scar-free spinal cord repair in neonatal mice
Abstract
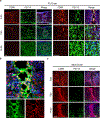
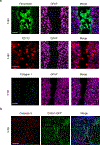
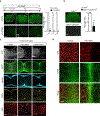
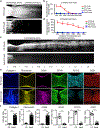
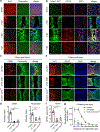
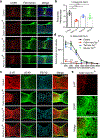
Spinal cord injury in mammals is thought to trigger scar formation with little regeneration of axons1-4. Here we show that a crush injury to the spinal cord in neonatal mice leads to scar-free healing that permits the growth of long projecting axons through the lesion. Depletion of microglia in neonatal mice disrupts this healing process and stalls the regrowth of axons, suggesting that microglia are critical for orchestrating the injury response. Using single-cell RNA sequencing and functional analyses, we find that neonatal microglia are transiently activated and have at least two key roles in scar-free healing. First, they transiently secrete fibronectin and its binding proteins to form bridges of extracellular matrix that ligate the severed ends of the spinal cord. Second, neonatal-but not adult-microglia express several extracellular and intracellular peptidase inhibitors, as well as other molecules that are involved in resolving inflammation. We transplanted either neonatal microglia or adult microglia treated with peptidase inhibitors into spinal cord lesions of adult mice, and found that both types of microglia significantly improved healing and axon regrowth. Together, our results reveal the cellular and molecular basis of the nearly complete recovery of neonatal mice after spinal cord injury, and suggest strategies that could be used to facilitate scar-free healing in the adult mammalian nervous system.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

