Relatives of rubella virus in diverse mammals
- PMID: 33029010
- PMCID: PMC7572621
- DOI: 10.1038/s41586-020-2812-9
Relatives of rubella virus in diverse mammals
Erratum in
-
Author Correction: Relatives of rubella virus in diverse mammals.Nature. 2020 Dec;588(7836):E2. doi: 10.1038/s41586-020-2897-1. Nature. 2020. PMID: 33199919
Abstract
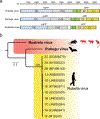
Since 1814, when rubella was first described, the origins of the disease and its causative agent, rubella virus (Matonaviridae: Rubivirus), have remained unclear1. Here we describe ruhugu virus and rustrela virus in Africa and Europe, respectively, which are, to our knowledge, the first known relatives of rubella virus. Ruhugu virus, which is the closest relative of rubella virus, was found in apparently healthy cyclops leaf-nosed bats (Hipposideros cyclops) in Uganda. Rustrela virus, which is an outgroup to the clade that comprises rubella and ruhugu viruses, was found in acutely encephalitic placental and marsupial animals at a zoo in Germany and in wild yellow-necked field mice (Apodemus flavicollis) at and near the zoo. Ruhugu and rustrela viruses share an identical genomic architecture with rubella virus2,3. The amino acid sequences of four putative B cell epitopes in the fusion (E1) protein of the rubella, ruhugu and rustrela viruses and two putative T cell epitopes in the capsid protein of the rubella and ruhugu viruses are moderately to highly conserved4-6. Modelling of E1 homotrimers in the post-fusion state predicts that ruhugu and rubella viruses have a similar capacity for fusion with the host-cell membrane5. Together, these findings show that some members of the family Matonaviridae can cross substantial barriers between host species and that rubella virus probably has a zoonotic origin. Our findings raise concerns about future zoonotic transmission of rubella-like viruses, but will facilitate comparative studies and animal models of rubella and congenital rubella syndrome.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

