Population-Wide Duchenne Muscular Dystrophy Carrier Detection by CK and Molecular Testing
- PMID: 33029525
- PMCID: PMC7537677
- DOI: 10.1155/2020/8396429
Population-Wide Duchenne Muscular Dystrophy Carrier Detection by CK and Molecular Testing
Abstract
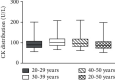
Carrier screening of Duchenne muscular dystrophy (DMD) has not been widely evaluated. To identify definite DMD female carriers prior to or in early pregnancy, we studied a large population of reproductive age females and provided informed reproductive options to DMD carriers. 37268 females were recruited from the Hangzhou Family Planning Publicity and Technology Guidance Station/Hangzhou Health Service Center for Children and Women, Hangzhou, China, between October 10, 2017, and December 16, 2018. CK activity was measured with follow-up serum DMD genetic testing in subjects with hyperCKemia, defined as CK > 200 U/L. The calculated upper reference limit (97.5th percentile) of serum creatine kinase (CK) for females aged 20-50 years in this study was near the reference limit recommended by the manufacturer (200 U/L), above which was defined as hyperCKemia. 427 females (1.2%) harbored initially elevated CK, among which 281 females (response rate of 65.8%) accepted CK retesting. DMD genetic testing was conducted on 62 subjects with sustained serum CK > 200 U/L and 16 females with a family history of DMD. Finally, 6 subjects were confirmed to be DMD definite carriers. The estimated DMD female carrier rate in this study was 1 : 4088 (adjusting for response rate), an underestimated rate, since only 50% to 70% of DMD female carriers manifest elevated serum CK, and carriers in this study may have been missed due to lack of follow-up or inability to detect all DMD pathogenic variants by current genetic testing.
Copyright © 2020 Shuai Han et al.
Conflict of interest statement
The University of Rochester receives research support for Dr. Griggs' research from both PTC Therapeutics and Sarepta Pharmaceuticals. Dr. Griggs received compensation for serving as Chair of a Data Safety Monitoring Board for PTC Therapeutics, Idera Pharmaceuticals, and Solid Bioscience. Dr. Griggs received personal compensation and support for travel for an Advisory Committee meeting from Sarepta Pharmaceuticals. Dr. Griggs receives compensation as a consultant and speaker from Strongbridge Pharmaceuticals. Dr. Griggs receives compensation as a consultant for Steal Pharmaceuticals. Dr. Griggs receives research grants from the NIH, the MDA and the Parent Project for Muscular Dystrophy for work on Duchesne muscular dystrophy. The remaining authors report no disclosures.
Figures



Similar articles
-
[Carrier screening model for Duchenne muscular dystrophy for women of reproductive age based on a pre-pregnancy birth defect control platform].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2021 May 10;38(5):485-487. doi: 10.3760/cma.j.cn511374-20200331-00223. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2021. PMID: 33974262 Chinese.
-
Underlying diseases in sporadic presentation of high creatine kinase levels in girls.Clin Chim Acta. 2021 Aug;519:198-203. doi: 10.1016/j.cca.2021.05.003. Epub 2021 May 7. Clin Chim Acta. 2021. PMID: 33965408
-
Newborn screening and genomic analysis of duchenne muscular dystrophy in Henan, China.Clin Chim Acta. 2023 Jan 15;539:90-96. doi: 10.1016/j.cca.2022.11.024. Epub 2022 Dec 11. Clin Chim Acta. 2023. PMID: 36516925
-
Newborn screening for Duchenne muscular dystrophy-early detection and diagnostic algorithm for female carriers of Duchenne muscular dystrophy.Am J Med Genet C Semin Med Genet. 2022 Jun;190(2):197-205. doi: 10.1002/ajmg.c.32000. Epub 2022 Sep 24. Am J Med Genet C Semin Med Genet. 2022. PMID: 36152336 Free PMC article. Review.
-
Successful Pregnancy Outcome With Preconception Care in a Symptomatic Carrier of Duchenne Muscular Dystrophy: Case Report and Literature Review.Am J Med Genet A. 2025 Mar;197(3):e63926. doi: 10.1002/ajmg.a.63926. Epub 2024 Oct 31. Am J Med Genet A. 2025. PMID: 39482265 Review.
Cited by
-
miRNome profiling in Duchenne muscular dystrophy; identification of asymptomatic and manifesting female carriers.Biosci Rep. 2021 Sep 30;41(9):BSR20211325. doi: 10.1042/BSR20211325. Biosci Rep. 2021. PMID: 34472584 Free PMC article.
-
The Clinical Development of Taldefgrobep Alfa: An Anti-Myostatin Adnectin for the Treatment of Duchenne Muscular Dystrophy.Neurol Ther. 2024 Feb;13(1):183-219. doi: 10.1007/s40120-023-00570-w. Epub 2024 Jan 8. Neurol Ther. 2024. PMID: 38190001 Free PMC article.
-
Expression of SRP-9001 dystrophin and stabilization of motor function up to 2 years post-treatment with delandistrogene moxeparvovec gene therapy in individuals with Duchenne muscular dystrophy.Front Cell Dev Biol. 2023 Jul 11;11:1167762. doi: 10.3389/fcell.2023.1167762. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37497476 Free PMC article.
-
Healthcare Stakeholder Perspectives on a Value Assessment Approach for Duchenne Muscular Dystrophy Therapies.J Multidiscip Healthc. 2024 Aug 29;17:4199-4212. doi: 10.2147/JMDH.S458181. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 39224484 Free PMC article.
-
Echocardiographic signs of subclinical cardiac function impairment in Duchenne dystrophy gene carriers.Sci Rep. 2020 Nov 27;10(1):20794. doi: 10.1038/s41598-020-77882-6. Sci Rep. 2020. PMID: 33247228 Free PMC article.
References
-
- Sumita D. R., Vainzof M., Campiotto S., et al. Absence of correlation between skewed X inactivation in blood and serum creatine-kinase levels in Duchenne/Becker female carriers. American Journal of Medical Genetics. 1998;80(4):356–361. doi: 10.1002/(SICI)1096-8628(19981204)80:4<356::AID-AJMG10>3.0.CO;2-O. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

