Genetic causes of growth hormone insensitivity beyond GHR
- PMID: 33029712
- PMCID: PMC7979432
- DOI: 10.1007/s11154-020-09603-3
Genetic causes of growth hormone insensitivity beyond GHR
Abstract
Growth hormone insensitivity (GHI) syndrome, first described in 1966, is classically associated with monogenic defects in the GH receptor (GHR) gene which result in severe post-natal growth failure as consequences of insulin-like growth factor I (IGF-I) deficiency. Over the years, recognition of other monogenic defects downstream of GHR has greatly expanded understanding of primary causes of GHI and growth retardation, with either IGF-I deficiency or IGF-I insensitivity as clinical outcomes. Mutations in IGF1 and signaling component STAT5B disrupt IGF-I production, while defects in IGFALS and PAPPA2, disrupt transport and release of circulating IGF-I, respectively, affecting bioavailability of the growth-promoting IGF-I. Defects in IGF1R, cognate cell-surface receptor for IGF-I, disrupt not only IGF-I actions, but actions of the related IGF-II peptides. The importance of IGF-II for normal developmental growth is emphasized with recent identification of defects in the maternally imprinted IGF2 gene. Current application of next-generation genomic sequencing has expedited the pace of identifying new molecular defects in known genes or in new genes, thereby expanding the spectrum of GH and IGF insensitivity. This review discusses insights gained and future directions from patient-based molecular and functional studies.
Keywords: GH insensitivity; GH-IGF-I axis; IGF-I deficiency; IGF-I insensitivity.
Conflict of interest statement
Figures




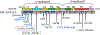

References
-
- Rosenfeld RG. Biochemical diagnostic strategies in the evaluation of short stature: the diagnosis of insulin-like growth factor deficiency. Horm Res. 1996;46(4–5):170–3. - PubMed
-
- Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone--a new inborn error of metabolism? Isr J Med Sci. 1966;2(2):152–5. - PubMed
-
- Goncalves FT, Fridman C, Pinto EM, Guevara-Aguirre J, Shevah O, Rosembloom AL, et al. The E180splice mutation in the GHR gene causing Laron syndrome: witness of a Sephardic Jewish exodus from the Iberian Peninsula to the New World? American journal of medical genetics Part A. 2014;164A(5):1204–8. - PubMed
-
- Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15(3):369–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

