Safety, Tolerability, and Pharmacokinetics of Mevidalen (LY3154207), a Centrally Acting Dopamine D1 Receptor-Positive Allosteric Modulator (D1PAM), in Healthy Subjects
- PMID: 33029934
- PMCID: PMC8048550
- DOI: 10.1002/cpdd.874
Safety, Tolerability, and Pharmacokinetics of Mevidalen (LY3154207), a Centrally Acting Dopamine D1 Receptor-Positive Allosteric Modulator (D1PAM), in Healthy Subjects
Abstract
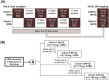
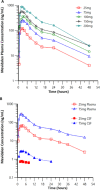
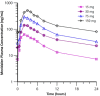
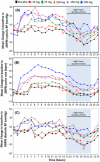
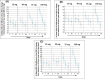
Activation of the brain dopamine D1 receptor has attracted attention because of its promising role in neuropsychiatric diseases. Although efforts to develop D1 agonists have been challenging, a positive allosteric modulator (PAM), represents an attractive approach with potential better drug-like properties. Phase 1 single-ascending-dose (SAD; NCT03616795) and multiple-ascending-dose (MAD; NCT02562768) studies with the D1PAM mevidalen (LY3154207) were conducted with healthy subjects. There were no treatment-related serious adverse events (AEs) in these studies. In the SAD study, 25-200 mg administered orally showed dose-proportional pharmacokinetics (PK) and acute dose-related increases in systolic blood pressure (SBP) and diastolic blood pressure DBP) and pulse rate at doses ≥ 75 mg. AE related to central activation were seen at doses ≥ 75 mg. At 25 and 75 mg, central penetration of mevidalen was confirmed by measurement of mevidalen in cerebrospinal fluid. In the MAD study, once-daily doses of mevidalen at 15-150 mg for 14 days showed dose-proportional PK. Acute dose-dependent increases in SBP, DBP, and PR were observed on initial administration, but with repeated dosing the effects diminished and returned toward baseline levels. Overall, these findings support further investigation of mevidalen as a potential treatment for a range of neuropsychiatric disorders.
Trial registration: ClinicalTrials.gov NCT03305809 NCT03616795 NCT02562768.
Keywords: dopamine; mevidalen (LY3154207); pharmacokinetics; safety; tolerability.
© 2020 Eli Lilly and Company. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Darren Wilbraham, Kevin M. Biglan, Kjell A. Svensson, Max Tsai, and William Kielbasa are employees of Eli Lilly and Company Inc., and may own stock in this company.
Figures
References
-
- Svensson KA, Hao J, Bruns RF. Positive Allosteric modulators of the dopamine D1 receptor: a new mechanism for the treatment of neuropsychiatric disorders. In: Witkin JM, ed. Advances in Pharmacology. Academic Press; 2019;86:273‐305. - PubMed
-
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377‐1384. - PubMed
-
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30(5):188‐193. - PubMed
-
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182‐217. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

