Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence
- PMID: 33030400
- PMCID: PMC8496707
- DOI: 10.1080/15548627.2020.1833515
Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence
Abstract
Deficiency in decidualization has been widely regarded as an important cause of spontaneous abortion. Generalized decidualization also includes massive infiltration and enrichment of NK cells. However, the underlying mechanism of decidual NK (dNK) cell residence remains largely unknown. Here, we observe that the increased macroautophagy/autophagy of decidual stromal cells (DSCs) during decidualization, facilitates the adhesion and retention of dNK cells during normal pregnancy. Mechanistically, this process is mediated through activation of the MITF-TNFRSF14/HVEM signaling, and further upregulation of multiple adhesion adhesions (e.g. Selectins and ICAMs) in a MMP9-dependent manner. Patients with unexplained spontaneous abortion display insufficient DSC autophagy and dNK cell residence. In addition, poor vascular remodeling of placenta, low implantation number and high ratio of embryo loss are observed in NK cell depletion mice. In therapeutic studies, low doses of rapamycin, a known autophagy inducer that significantly promotes endometrium autophagy and NK cell residence, and improves embryo absorption in spontaneous abortion mice models, which should be dependent on the activation of MITF-TNFRSF14/HVEM-MMP9-adhension molecules axis. This observation reveals novel molecular mechanisms underlying DSCs autophagy-driven dNK cell residence, and provides a potential therapeutic strategy to prevent spontaneous abortion.Abbreviations: ACTA2/αSMA: actin alpha 2, smooth muscle; ATG: autophagy-related; ATG5over ESC: ATG5-overexpressed ESCs; BTLA: B and T lymphocyte associated; CDH1: cadherin 1; CDH5: cadherin 5; CXCL12: C-X-C motif chemokine ligand 12; dNK: decidual NK; DIC: decidual immune cell; DSC: decidual stromal cell; EOMES: eomesodermin; ESC: endometrial stromal cell; FCGR3A/CD16: Fc fragment of IgG receptor IIIa; HUVEC: human umbilical vein endothelial cell; ICAM: intercellular cell adhesion molecule; ILC: innate lymphoid cell; ITGB1: integrin subunit beta 1; ITGA2: integrin subunit alpha 2; IPA: Ingenuity Pathway Analysis; KIR2DL1: killer cell immunoglobulin like receptor, two Ig domains and long cytoplasmic tail 1; KLRD1/CD94: killer cell lectin like receptor D1; KLRK1/NKG2D: killer cell lectin like receptor K1; MAP1LC3B/LC3B: microtubule associated protein 1 light chain 3 beta; 3-MA: 3-methyladenine; MITF: melanocyte inducing transcription factor; MiT-TFE: microphthalmia family of bHLH-LZ transcription factors; MMP9: matrix metalloproteinase 9; MTOR: mechanistic target of rapamycin kinase; NCAM1/CD56: neural cell adhesion molecule 1; NCR2/NKp44: natural cytotoxicity triggering receptor 2; NK: natural killer; KLRB1/NK1.1: killer cell lectin like receptor B1; NP: normal pregnancy; PBMC: peripheral blood mononuclear cell; PECAM1/CD31: platelet and endothelial cell adhesion molecule 1; pNK: peripheral blood NK; PRF1/Perforin: Perforin 1; PTPRC/CD45: protein tyrosine phosphatase receptor type C; Rapa: rapamycin; rh-TNFSF14/LIGHT: recombinant human TNFSF14/LIGHT; SA: spontaneous abortion; SELE: selectin E; SELP: selectin P; SELL: selectin L; siATG5 DSCs: ATG5-silenced DSCs; siTNFRSF14/HVEM DSCs: TNFRSF14/HVEM-silenced DSCs; TBX21/T-bet: T-box transcription factor 21; SQSTM1/p62: sequestosome 1; TNFRSF14/HVEM: TNF receptor superfamily member 14; TNFSF14/LIGHT: TNF superfamily member 14; uNK: uterine NK; UIC: uterine immune cell; USC: uterine stromal cell; VCAM1: vascular cell adhesion molecule 1; VIM: vimentin.
Keywords: Autophagy; MITF; MMP9; NK cells; TNFRSF14/HVEM; abortion; decidual stromal cells; early pregnancy; residence.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
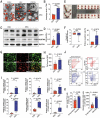
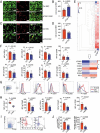
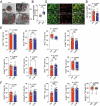

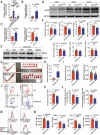
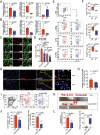
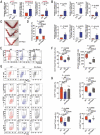
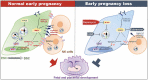
References
-
- Weselak M, Arbuckle TE, Walker MC, et al. The influence of the environment and other exogenous agents on spontaneous abortion risk. J Toxicol Environ Health B Crit Rev. 2008;11:221–241. - PubMed
-
- Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125:1042–1048. - PubMed
-
- Gellersen B, Brosens JJ.. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. - PubMed
-
- Lucas ES, Dyer NP, Murakami K, et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells. 2016;34:346–356. - PubMed
-
- Kajihara T, Tanaka K, Oguro T, et al. Androgens modulate the morphological characteristics of human endometrial stromal cells decidualized in vitro. Reprod Sci. 2014;21:372–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
