The Opioid Epidemic Within the COVID-19 Pandemic: Drug Testing in 2020
- PMID: 33031013
- PMCID: PMC7875135
- DOI: 10.1089/pop.2020.0230
The Opioid Epidemic Within the COVID-19 Pandemic: Drug Testing in 2020
Abstract
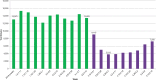
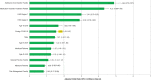
The convergence of the opioid epidemic and the coronavirus disease 2019 (COVID-19) pandemic has created new health care challenges. The authors analyzed changes in clinical drug testing patterns and results at a national clinical laboratory, comparing data obtained before and during the pandemic. Testing for prescription and illicit drugs declined rapidly during the pandemic, with weekly test volumes falling by approximately 70% from the baseline period to the trough (the week beginning March 29) before rising in subsequent weeks. Among individuals tested, positivity increased by 35% for non-prescribed fentanyl and 44% for heroin during the pandemic. Positivity for non-prescribed fentanyl increased significantly among patients positive for other drugs: by 89% for specimens positive for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < 0.01 for all comparisons). These findings suggest significant increases in dangerous drug combinations. Positivity for non-prescribed use of many other drugs remained consistent or declined for some drugs, relative to pre-pandemic patterns. Models adjusting for potential confounding variables, including medication-assisted treatment and treatment at a substance use disorder facility indicated that the risk for non-prescribed fentanyl positivity rose by more than 50% during the pandemic. In summary, these findings demonstrate decreased drug testing overall, with increased positivity for high-risk drugs and dangerous drug combinations. The convergence of the drug abuse epidemic and COVID-19 pandemic has led to an increased need for health care and public health resources dedicated to supporting vulnerable patients and addressing the underlying causes of these disturbing trends.
Keywords: COVID-19; SARS-CoV-2; clinical drug testing; fentanyl; opioid; substance use disorder.
Conflict of interest statement
Quest Diagnostics provided support in the form of salaries for Dr. Kaufman, Mr. Niles, and Mr. Radcliff and for Dr. Gudin in the form of consulting fees. Dr. Kaufman and Mr. Radcliff hold stock in Quest Diagnostics.
Figures



References
-
- Centers for Disease Control and Prevention. Opioid overdose. https://www.cdc.gov/drugoverdose/data/index.html Accessed July28, 2020
-
- Centers for Disease Control and Prevention. National Center for Health Statistics. Data Brief 356. Overdose death in the United States, 1999–2018. https://www.cdc.gov/nchs/data/databriefs/db356_tables-508.pdf#1 Accessed July28, 2020
-
- Centers for Disease Control and Prevention. National Center for Health Statistics Vital statistics rapid release, provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Accessed July28, 2020
-
- Centers for Disease Control and Prevention. Framework for healthcare systems providing non-COVID-19 clinical care during the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/framework-non-COVID-care.html Accessed July28, 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
