The procoagulant activity of tissue factor expressed on fibroblasts is increased by tissue factor-negative extracellular vesicles
- PMID: 33031441
- PMCID: PMC7544082
- DOI: 10.1371/journal.pone.0240189
The procoagulant activity of tissue factor expressed on fibroblasts is increased by tissue factor-negative extracellular vesicles
Abstract
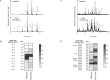
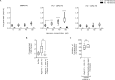
Tissue factor (TF) is critical for the activation of blood coagulation. TF function is regulated by the amount of externalised phosphatidylserine (PS) and phosphatidylethanolamine (PE) on the surface of the cell in which it is expressed. We investigated the role PS and PE in fibroblast TF function. Fibroblasts expressed 6-9 x 104 TF molecules/cell but had low specific activity for FXa generation. We confirmed that this was associated with minimal externalized PS and PE and characterised for the first time the molecular species of PS/PE demonstrating that these differed from those found in platelets. Mechanical damage of fibroblasts, used to simulate vascular injury, increased externalized PS/PE and led to a 7-fold increase in FXa generation that was inhibited by annexin V and an anti-TF antibody. Platelet-derived extracellular vesicles (EVs), that did not express TF, supported minimal FVIIa-dependent FXa generation but substantially increased fibroblast TF activity. This enhancement in fibroblast TF activity could also be achieved using synthetic liposomes comprising 10% PS without TF. In conclusion, despite high levels of surface TF expression, healthy fibroblasts express low levels of external-facing PS and PE limiting their ability to generate FXa. Addition of platelet-derived TF-negative EVs or artificial liposomes enhanced fibroblast TF activity in a PS dependent manner. These findings contribute information about the mechanisms that control TF function in the fibroblast membrane.
Conflict of interest statement
No authors have competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

