An Amygdala Circuit Mediates Experience-Dependent Momentary Arrests during Exploration
- PMID: 33031743
- PMCID: PMC8276519
- DOI: 10.1016/j.cell.2020.09.023
An Amygdala Circuit Mediates Experience-Dependent Momentary Arrests during Exploration
Abstract
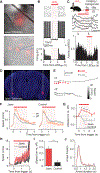
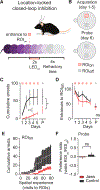
Exploration of novel environments ensures survival and evolutionary fitness. It is expressed through exploratory bouts and arrests that change dynamically based on experience. Neural circuits mediating exploratory behavior should therefore integrate experience and use it to select the proper behavioral output. Using a spatial exploration assay, we uncovered an experience-dependent increase in momentary arrests in locations where animals arrested previously. Calcium imaging in freely exploring mice revealed a genetically and projection-defined neuronal ensemble in the basolateral amygdala that is active during self-paced behavioral arrests. This ensemble was recruited in an experience-dependent manner, and closed-loop optogenetic manipulation of these neurons revealed that they are sufficient and necessary to drive experience-dependent arrests during exploration. Projection-specific imaging and optogenetic experiments revealed that these arrests are effected by basolateral amygdala neurons projecting to the central amygdala, uncovering an amygdala circuit that mediates momentary arrests in familiar places but not avoidance or anxiety/fear-like behaviors.
Keywords: amygdala; experience; exploration; familiarity; latent learning; momentary arrest; movement; novelty.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



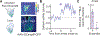

Comment in
-
Moments of exploration.Nat Rev Neurosci. 2020 Dec;21(12):666-667. doi: 10.1038/s41583-020-00403-x. Nat Rev Neurosci. 2020. PMID: 33110223 No abstract available.
References
-
- Benjamini Y, Lipkind D, Horev G, Fonio E, Kafkafi N, and Golani I. (2010). Ten ways to improve the quality of descriptions of whole-animal movement. Neurosci. Biobehav. Rev 34, 1351–1365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

