Targeting metabolic pathways for extension of lifespan and healthspan across multiple species
- PMID: 33031925
- PMCID: PMC9038119
- DOI: 10.1016/j.arr.2020.101188
Targeting metabolic pathways for extension of lifespan and healthspan across multiple species
Abstract
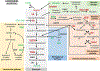
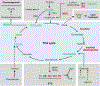
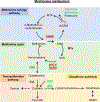
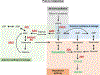
Metabolism plays a significant role in the regulation of aging at different levels, and metabolic reprogramming represents a major driving force in aging. Metabolic reprogramming leads to impaired organismal fitness, an age-dependent increase in susceptibility to diseases, decreased ability to mount a stress response, and increased frailty. The complexity of age-dependent metabolic reprogramming comes from the multitude of levels on which metabolic changes can be connected to aging and regulation of lifespan. This is further complicated by the different metabolic requirements of various tissues, cross-organ communication via metabolite secretion, and direct effects of metabolites on epigenetic state and redox regulation; however, not all of these changes are causative to aging. Studies in yeast, flies, worms, and mice have played a crucial role in identifying mechanistic links between observed changes in various metabolic traits and their effects on lifespan. Here, we review how changes in the organismal and organ-specific metabolome are associated with aging and how targeting of any one of over a hundred different targets in specific metabolic pathways can extend lifespan. An important corollary is that restriction or supplementation of different metabolites can change activity of these metabolic pathways in ways that improve healthspan and extend lifespan in different organisms. Due to the high levels of conservation of metabolism in general, translating findings from model systems to human beings will allow for the development of effective strategies for human health- and lifespan extension.
Keywords: Aging; C. elegans; Drosophila; Metabolism; Mice; Yeast.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no declarations of interest.
Figures


References
-
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C, 2005. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp. Gerontol. 40, 685–693. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

