TOCIVID-19 - A multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia. Study protocol
- PMID: 33031955
- PMCID: PMC7536129
- DOI: 10.1016/j.cct.2020.106165
TOCIVID-19 - A multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia. Study protocol
Abstract
Background: Pneumonia is the most frequent complication of COVID-19, due to an aberrant host immune response that is associated with an acute respiratory distress syndrome, and, in most critical patients, with a "cytokine storm". IL-6 might play a key role in the cytokine storm and might be a potential target to treat severe and critical COVID-19. Tocilizumab is a recombinant humanized monoclonal antibody, directed against IL-6 receptor.
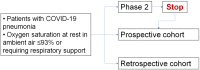
Methods: This multicentre study project includes a single-arm phase 2 study and a further parallel cohort, enrolling hospitalized patients with COVID-19 pneumonia and oxygen saturation at rest in ambient air ≤93% or requiring respiratory support. Patients receive tocilizumab 8 mg/kg (up to 800 mg) as one intravenous administration. A second administration (same dose) after 12 h is optional. Two-week and one-month lethality rates are the co-primary endpoints. Sample size planned for the phase 2 study is 330 patients. The parallel cohort will include patients who cannot enter the phase 2 study because being intubated from more than 24 h, or having already received tocilizumab, or the phase 2 study has reached sample size. Primary analysis will include patients enrolled in the phase 2 study. Results of the primary analysis will be validated in the prospective cohort of patients consecutively registered after phase 2 closure from March 20 to March 24, who were potentially eligible for the phase 2 study.
Conclusion: This trial aims to verify the safety and efficacy of tocilizumab in the Italian population with COVID-19 pneumonia and respiratory impairment. EudraCT Number: 2020-001110-38; Clinicaltrials.gov ID NCT04317092.
Keywords: COVID-19 pneumonia; Phase 2 study; Tocilizumab.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
AMM, RP, PP, LA, MCa, MCo, GD, NCF, FF, MM, VM, CM, EAN, PC, and CG have no competing interests. PA has received fee for advisory/consultant role and research funds from Roche. CS has received consulting fees (less than $10,000) and research support from Roche. FP and MCP coordinate three academic clinical trials in oncology, promoted by the Istituto Nazionale Tumori di Napoli, that are supported by Roche (
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical