Involvement of the mitochondrial nuclease EndoG in the regulation of cell proliferation through the control of reactive oxygen species
- PMID: 33032073
- PMCID: PMC7552104
- DOI: 10.1016/j.redox.2020.101736
Involvement of the mitochondrial nuclease EndoG in the regulation of cell proliferation through the control of reactive oxygen species
Abstract
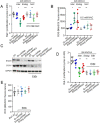
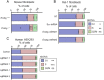
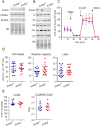
The apoptotic nuclease EndoG is involved in mitochondrial DNA replication. Previous results suggested that, in addition to regulate cardiomyocyte hypertrophy, EndoG could be involved in cell proliferation. Here, by using in vivo and cell culture models, we investigated the role of EndoG in cell proliferation. Genetic deletion of Endog both in vivo and in cultured cells or Endog silencing in vitro induced a defect in rodent and human cell proliferation with a tendency of cells to accumulate in the G1 phase of cell cycle and increased reactive oxygen species (ROS) production. The defect in cell proliferation occurred with a decrease in the activity of the AKT/PKB-GSK-3β-Cyclin D axis and was reversed by addition of ROS scavengers. EndoG deficiency did not affect the expression of ROS detoxifying enzymes, nor the expression of the electron transport chain complexes and oxygen consumption rate. Addition of the micropeptide Humanin to EndoG-deficient cells restored AKT phosphorylation and proliferation without lowering ROS levels. Thus, our results show that EndoG is important for cell proliferation through the control of ROS and that Humanin can restore cell division in EndoG-deficient cells and counteracts the effects of ROS on AKT phosphorylation.
Keywords: Cell proliferation; Cell signaling; EndoG; Humanin; Mitochondria; Reactive oxygen species; Romo1.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures




References
-
- Sakamaki T., Casimiro M.C., Ju X., Quong A.A., Katiyar S., Liu M., Jiao X., Li A., Zhang X., Lu Y., Wang C., Byers S., Nicholson R., Link T., Shemluck M., Yang J., Fricke S.T., Novikoff P.M., Papanikolaou A., Arnold A., Albanese C., Pestell R. Cyclin D1 determines mitochondrial function in vivo. Mol. Cell Biol. 2006;26:5449–5469. doi: 10.1128/MCB.02074-05. - DOI - PMC - PubMed
-
- Wang Z., Fan M., Candas D., Zhang T.Q., Qin L., Eldridge A., Wachsmann-Hogiu S., Ahmed K.M., Chromy B.A., Nantajit D., Duru N., He F., Chen M., Finkel T., Weinstein L.S., Li J.J. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev. Cell. 2014;29:217–232. doi: 10.1016/j.devcel.2014.03.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

