Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection
- PMID: 33032297
- PMCID: PMC7672690
- DOI: 10.1038/s41586-020-2838-z
Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection
Abstract
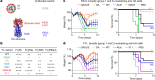
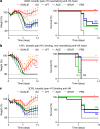
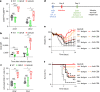
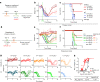
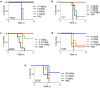
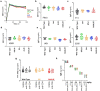
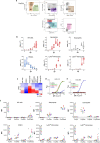
Antibodies against viral pathogens represent promising therapeutic agents for the control of infection, and their antiviral efficacy has been shown to require the coordinated function of both the Fab and Fc domains1. The Fc domain engages a wide spectrum of receptors on discrete cells of the immune system to trigger the clearance of viruses and subsequent killing of infected cells1-4. Here we report that Fc engineering of anti-influenza IgG monoclonal antibodies for selective binding to the activating Fcγ receptor FcγRIIa results in enhanced ability to prevent or treat lethal viral respiratory infection in mice, with increased maturation of dendritic cells and the induction of protective CD8+ T cell responses. These findings highlight the capacity for IgG antibodies to induce protective adaptive immunity to viral infection when they selectively activate a dendritic cell and T cell pathway, with important implications for the development of therapeutic antibodies with improved antiviral efficacy against viral respiratory pathogens.
Conflict of interest statement
S.B. and J.V.R. are inventors on a patent (WO2019125846A1) describing the GAALIE variant and its use in therapeutic monoclonal antibodies; D.C. and H.W.V. are employees of Vir Biotechnology Inc. and may hold shares in Vir Biotechnology Inc.; J.V.R. is a member of the scientific advisory board and a consultant of Vir Biotechnology Inc.
Figures







Comment in
-
Engineered antibodies to combat viral threats.Nature. 2020 Dec;588(7838):398-399. doi: 10.1038/d41586-020-03196-2. Nature. 2020. PMID: 33208906 No abstract available.
-
Varying the Constant: Mechanisms of Fc-Mediated Immunity to Influenza Virus.Cell Host Microbe. 2020 Dec 9;28(6):769-770. doi: 10.1016/j.chom.2020.11.009. Cell Host Microbe. 2020. PMID: 33301713
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

