The Seattle Flu Study: a multiarm community-based prospective study protocol for assessing influenza prevalence, transmission and genomic epidemiology
- PMID: 33033018
- PMCID: PMC7542952
- DOI: 10.1136/bmjopen-2020-037295
The Seattle Flu Study: a multiarm community-based prospective study protocol for assessing influenza prevalence, transmission and genomic epidemiology
Abstract
Introduction: Influenza epidemics and pandemics cause significant morbidity and mortality. An effective response to a potential pandemic requires the infrastructure to rapidly detect, characterise, and potentially contain new and emerging influenza strains at both an individual and population level. The objective of this study is to use data gathered simultaneously from community and hospital sites to develop a model of how influenza enters and spreads in a population.
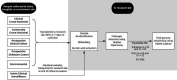
Methods and analysis: Starting in the 2018-2019 season, we have been enrolling individuals with acute respiratory illness from community sites throughout the Seattle metropolitan area, including clinics, childcare facilities, Seattle-Tacoma International Airport, workplaces, college campuses and homeless shelters. At these sites, we collect clinical data and mid-nasal swabs from individuals with at least two acute respiratory symptoms. Additionally, we collect residual nasal swabs and data from individuals who seek care for respiratory symptoms at four regional hospitals. Samples are tested using a multiplex molecular assay, and influenza whole genome sequencing is performed for samples with influenza detected. Geospatial mapping and computational modelling platforms are in development to characterise the regional spread of influenza and other respiratory pathogens.
Ethics and dissemination: The study was approved by the University of Washington's Institutional Review Board (STUDY00006181). Results will be disseminated through talks at conferences, peer-reviewed publications and on the study website (www.seattleflu.org).
Keywords: influenza; protocol; respiratory infection; surveillance; virology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HYC receives research support from Sanofi, Cepheid and Genentech/Roche and is a consultant for Merck. JAE receives research support to her institution from AstraZeneca, GlaxoSmithKline, Merck and Novavax and is a consultant for Sanofi Pasteur and Meissa Vaccines.
Figures


References
-
- Prevention CfDCa Disease burden of influenza 2020. Available: https://www.cdc.gov/flu/about/burden/index.html [Accessed 1 Oct 2020].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
