Vedolizumab treatment across antiretroviral treatment interruption in chronic HIV infection: the HAVARTI protocol for a pilot dose-ranging clinical trial to assess safety, tolerance, immunological and virological activity
- PMID: 33033101
- PMCID: PMC7545629
- DOI: 10.1136/bmjopen-2020-041359
Vedolizumab treatment across antiretroviral treatment interruption in chronic HIV infection: the HAVARTI protocol for a pilot dose-ranging clinical trial to assess safety, tolerance, immunological and virological activity
Abstract
Introduction: Continuous antiretroviral therapy (ART) suppresses HIV plasma viral load (pVL) to very low levels, which allows for some immune recovery. Discontinuation of ART leads to pVL rebound from reservoirs of persistence and latency, and progressive immunodeficiency. One promising but controversial strategy targeting CD4+ T lymphocytes with a monoclonal antibody (mAb) against α4β7 integrin has shown promise through sustained virological remission of pVL (SVR) in SIV239-infected rhesus macaques. We propose to assess the safety and tolerability of vedolizumab, a licensed humanised mAb against human α4β7 integrin, in healthy HIV-infected adults on ART. This study will also assess, by analytical treatment interruption (ATI), whether vedolizumab treatment can induce SVR beyond ART and vedolizumab treatment.
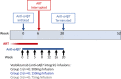
Methods and analysis: The HIV-ART-vedolizumab-ATI (HAVARTI) trial is a single-arm, dose-ranging pilot trial in healthy HIV-positive adult volunteers receiving ART. Twelve consenting persons will be enrolled in sequential groups of 4 to each serial dosing vedolizumab regimen (300 mg, 150 mg, 75 mg). The primary outcomes are: (1) to assess the safety and tolerability of seven serial infusions of vedolizumab at each of three doses; (2) to identify the immunovirological measures, including pVL and T-cell kinetics, that characterise HIV/ART cases before, during, after vedolizumab treatment and ATI; and (3) to seek SVR of pVL after ATI. Secondary outcomes will include immune reconstitution and pVL suppression as well as immune reconstitution and long-term safety following re-initiation of ART in the absence of SVR.
Ethics and dissemination: The study protocol was approved by the Ottawa Health Science Network-REB and by the Health Canada Therapeutic Products Directorate. A Data Safety Monitor will review safety information at regular intervals. The final manuscript will be submitted to an open access journal within a year of study completion.
Trial registration number: ClinicalTrials.gov NCT03147859; https://clinicaltrials.gov/ct2/show/NCT03147859.
Keywords: HIV & AIDS; clinical trials; immunology; inflammatory bowel disease.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
An open-label phase 1 clinical trial of the anti-α4β7 monoclonal antibody vedolizumab in HIV-infected individuals.Sci Transl Med. 2019 Sep 11;11(509):eaax3447. doi: 10.1126/scitranslmed.aax3447. Epub 2019 Sep 5. Sci Transl Med. 2019. PMID: 31488581 Clinical Trial.
-
Virological remission after antiretroviral therapy interruption in female African HIV seroconverters.AIDS. 2019 Feb 1;33(2):185-197. doi: 10.1097/QAD.0000000000002044. AIDS. 2019. PMID: 30325764
-
Phospholipid Metabolism Is Associated with Time to HIV Rebound upon Treatment Interruption.mBio. 2021 Feb 23;12(1):e03444-20. doi: 10.1128/mBio.03444-20. mBio. 2021. PMID: 33622719 Free PMC article.
-
Initiation of Antiretroviral Therapy during Primary HIV Infection: Effects on the Latent HIV Reservoir, Including on Analytic Treatment Interruptions.AIDS Rev. 2020 Oct 26;23(1):28-39. doi: 10.24875/AIDSRev.20000001. AIDS Rev. 2020. PMID: 33105471 Free PMC article.
-
The Role of Integrin α4β7 in HIV Pathogenesis and Treatment.Curr HIV/AIDS Rep. 2018 Apr;15(2):127-135. doi: 10.1007/s11904-018-0382-3. Curr HIV/AIDS Rep. 2018. PMID: 29478152 Free PMC article. Review.
Cited by
-
A Canadian Survey of Research on HIV-1 Latency-Where Are We Now and Where Are We Heading?Viruses. 2024 Feb 1;16(2):229. doi: 10.3390/v16020229. Viruses. 2024. PMID: 38400005 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
