Identification of Escherichia coli Host Genes That Influence the Bacteriophage Lambda (λ) T4 rII Exclusion (Rex) Phenotype
- PMID: 33033112
- PMCID: PMC7768251
- DOI: 10.1534/genetics.120.303643
Identification of Escherichia coli Host Genes That Influence the Bacteriophage Lambda (λ) T4 rII Exclusion (Rex) Phenotype
Abstract
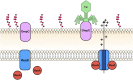
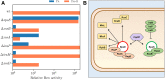
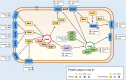
The T4rII exclusion (Rex) phenotype is the inability of T4rII mutant bacteriophage to propagate in hosts (Escherichia coli) lysogenized by bacteriophage lambda (λ). The Rex phenotype, triggered by T4rII infection of a rex+ λ lysogen, results in rapid membrane depolarization imposing a harsh cellular environment that resembles stationary phase. Rex "activation" has been proposed as an altruistic cell death system to protect the λ prophage and its host from T4rII superinfection. Although well studied for over 60 years, the mechanism behind Rex still remains unclear. We have identified key nonessential genes involved in this enigmatic exclusion system by examining T4rII infection across a collection of rex+ single-gene knockouts. We further developed a system for rapid, one-step isolation of host mutations that could attenuate/abrogate the Rex phenotype. For the first time, we identified host mutations that influence Rex activity and rex+ host sensitivity to T4rII infection. Among others, notable genes include tolA, ompA, ompF, ompW, ompX, ompT, lpp, mglC, and rpoS They are critical players in cellular osmotic balance and are part of the stationary phase and/or membrane distress regulons. Based on these findings, we propose a new model that connects Rex to the σS, σE regulons and key membrane proteins.
Keywords: Rex; bacteriophage T4rII; bacteriophage lambda; membrane proteins; sigma factors.
Copyright © 2020 by the Genetics Society of America.
Figures
Similar articles
-
A snapshot of the λ T4rII exclusion (Rex) phenotype in Escherichia coli.Curr Genet. 2021 Oct;67(5):739-745. doi: 10.1007/s00294-021-01183-2. Epub 2021 Apr 20. Curr Genet. 2021. PMID: 33877398 Review.
-
Polarity within pM and pE promoted phage lambda cI-rexA-rexB transcription and its suppression.Can J Microbiol. 2005 Jan;51(1):37-49. doi: 10.1139/w04-115. Can J Microbiol. 2005. PMID: 15782233
-
Over-expression of rexA nullifies T4rII exclusion in Escherichia coli K(lambda) lysogens.Can J Microbiol. 2004 Feb;50(2):133-6. doi: 10.1139/w03-115. Can J Microbiol. 2004. PMID: 15052316
-
Acquired mutations in phage lambda genes O or P that enable constitutive expression of a cryptic lambdaN+cI[Ts]cro- prophage in E. coli cells shifted from 30 degreesC to 42 degreesC, accompanied by loss of immlambda and Rex+ phenotypes and emergence of a non-immune exclusion-state.Gene. 1998 Nov 26;223(1-2):115-28. doi: 10.1016/s0378-1119(98)00363-1. Gene. 1998. PMID: 9858705
-
Interaction between bacteriophage lambda and its Escherichia coli host.Curr Opin Genet Dev. 1992 Oct;2(5):727-38. doi: 10.1016/s0959-437x(05)80133-9. Curr Opin Genet Dev. 1992. PMID: 1458022 Review.
Cited by
-
Intestinal phages interact with bacteria and are involved in human diseases.Gut Microbes. 2022 Jan-Dec;14(1):2113717. doi: 10.1080/19490976.2022.2113717. Gut Microbes. 2022. PMID: 36037202 Free PMC article. Review.
-
A snapshot of the λ T4rII exclusion (Rex) phenotype in Escherichia coli.Curr Genet. 2021 Oct;67(5):739-745. doi: 10.1007/s00294-021-01183-2. Epub 2021 Apr 20. Curr Genet. 2021. PMID: 33877398 Review.
References
-
- Apirakaramwong A., Fukuchi J., Kashiwagi K., Kakinuma Y., Ito E. et al. , 1998. Enhancement of cell death due to decrease in Mg2+uptake by OmpC (cation-selective porin) deficiency in ribosome modulation factor-deficient mutant. Biochem. Biophys. Res. Commun. 251: 482–487. 10.1006/bbrc.1998.9494 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

