Post-translational Regulation of DNA Polymerase η, a Connection to Damage-Induced Cohesion in Saccharomyces cerevisiae
- PMID: 33033113
- PMCID: PMC7768261
- DOI: 10.1534/genetics.120.303494
Post-translational Regulation of DNA Polymerase η, a Connection to Damage-Induced Cohesion in Saccharomyces cerevisiae
Abstract
Double-strand breaks that are induced postreplication trigger establishment of damage-induced cohesion in Saccharomyces cerevisiae, locally at the break site and genome-wide on undamaged chromosomes. The translesion synthesis polymerase, polymerase η, is required for generation of damage-induced cohesion genome-wide. However, its precise role and regulation in this process is unclear. Here, we investigated the possibility that the cyclin-dependent kinase Cdc28 and the acetyltransferase Eco1 modulate polymerase η activity. Through in vitro phosphorylation and structure modeling, we showed that polymerase η is an attractive substrate for Cdc28 Mutation of the putative Cdc28-phosphorylation site Ser14 to Ala not only affected polymerase η protein level, but also prevented generation of damage-induced cohesion in vivo We also demonstrated that Eco1 acetylated polymerase η in vitro Certain nonacetylatable polymerase η mutants showed reduced protein level, deficient nuclear accumulation, and increased ultraviolet irradiation sensitivity. In addition, we found that both Eco1 and subunits of the cohesin network are required for cell survival after ultraviolet irradiation. Our findings support functionally important Cdc28-mediated phosphorylation, as well as post-translational modifications of multiple lysine residues that modulate polymerase η activity, and provide new insights into understanding the regulation of polymerase η for damage-induced cohesion.
Keywords: Cdc28/Cdk1; Eco1; cohesin; damage-induced cohesion; polymerase eta.
Copyright © 2020 by the Genetics Society of America.
Figures


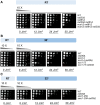
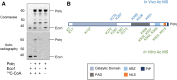
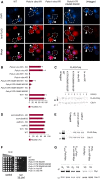

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

