Impact of age on the toxicity of immune checkpoint inhibition
- PMID: 33033183
- PMCID: PMC7545628
- DOI: 10.1136/jitc-2020-000871
Impact of age on the toxicity of immune checkpoint inhibition
Abstract
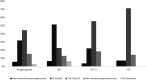
Indications for immune checkpoint inhibitor therapy are increasing. As the population ages, many patients receiving such drugs will be older adults. Such patients are under-represented in clinical trials, and therefore the safety of immune checkpoint inhibitors in this population has not been adequately assessed. A retrospective multicenter analysis of toxicities was performed in patients with advanced or metastatic solid cancers receiving anti-programmed cell death protein 1 (anti-PD-1) and/or anti-CTLA4 antibodies across three age cohorts (<65 years, 65-74 years and ≥75 years) using univariable and multivariable analyzes. Eligible patients (n=448) were divided into age cohorts: <65 years (n=185), 65-74 years (n=154) and ≥75 years (n=109). Fewer patients in the oldest cohort (7.3%) received an anti-CTLA4 antibody containing regimen compared with the younger cohorts (21.1% and 17.5%). There was no significant difference overall in all grade or ≥G3 toxicities between age cohorts. Significantly fewer patients in the older (65-74 years and ≥75 years) age cohorts discontinued treatment because of toxicity (10.1% and 7.4%) compared with in the <65 years cohort (20.5%; p=0.006). Using logistic regression, only treatment type (ipilimumab containing) was significantly associated with all grade toxicity. However, there was a significantly lower incidence of all-grade endocrine toxicity in the oldest cohort (11.0%) compared with the youngest cohort (22.7%, p=0.02; OR 0.43, 95% CI 0.21 to 0.87), while all-grade dermatological toxicity showed the reverse trend (28.4% vs 18.9%; OR 1.85, 95% CI 1.04 to 3.30). Results were corroborated in the sensitivity analysis using only data from patients who received PD-1 inhibitor monotherapy. This multicenter, real-world cohort demonstrates that immune checkpoint inhibitor therapy is safe and well tolerated regardless of age, with no appreciable increase in adverse events in older adult patients.
Keywords: immunotherapy; programmed cell death 1 receptor; self tolerance.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- UN . Ageing, 2019. Available: https://www.un.org/en/sections/issues-depth/ageing/
-
- Quinten C, Coens C, Ghislain I, et al. . The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European organisation for research and treatment of cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer 2015;51:2808–19. 10.1016/j.ejca.2015.08.027 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
