Reconstitution and visualization of HIV-1 capsid-dependent replication and integration in vitro
- PMID: 33033190
- PMCID: PMC8022914
- DOI: 10.1126/science.abc8420
Reconstitution and visualization of HIV-1 capsid-dependent replication and integration in vitro
Abstract
During the first half of the viral life cycle, HIV-1 reverse transcribes its RNA genome and integrates the double-stranded DNA copy into a host cell chromosome. Despite progress in characterizing and inhibiting these processes, in situ mechanistic and structural studies remain challenging. This is because these operations are executed by individual viral preintegration complexes deep within cells. We therefore reconstituted and imaged the early stages of HIV-1 replication in a cell-free system. HIV-1 cores released from permeabilized virions supported efficient, capsid-dependent endogenous reverse transcription to produce double-stranded DNA genomes, which sometimes looped out from ruptured capsid walls. Concerted integration of both viral DNA ends into a target plasmid then proceeded in a cell extract-dependent reaction. This reconstituted system uncovers the role of the capsid in templating replication.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
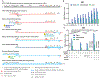
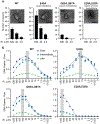
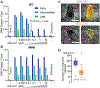

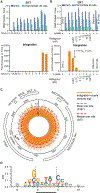
Comment in
-
Efficient HIV-1 in vitro reverse transcription: optimal capsid stability is required.Signal Transduct Target Ther. 2021 Jan 12;6(1):13. doi: 10.1038/s41392-020-00458-3. Signal Transduct Target Ther. 2021. PMID: 33436564 Free PMC article. No abstract available.
References
-
- De Clercq E, The nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors in the treatment of HIV infections (AIDS). Adv Pharmacol 67, 317–358 (2013). - PubMed
-
- Coffin JM, Fan H, The Discovery of Reverse Transcriptase. Annu Rev Virol 3, 29–51 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

