Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection
- PMID: 33033192
- PMCID: PMC7613218
- DOI: 10.1126/science.aba9301
Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection
Abstract
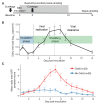
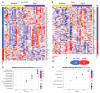
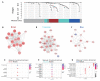
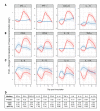
The variable outcome of viral exposure is only partially explained by known factors. We administered respiratory syncytial virus (RSV) to 58 volunteers, of whom 57% became infected. Mucosal neutrophil activation before exposure was highly predictive of symptomatic RSV disease. This was associated with a rapid, presymptomatic decline in mucosal interleukin-17A (IL-17A) and other mediators. Conversely, those who resisted infection showed presymptomatic activation of IL-17- and tumor necrosis factor-related pathways. Vulnerability to infection was not associated with baseline microbiome but was reproduced in mice by preinfection chemokine-driven airway recruitment of neutrophils, which caused enhanced disease mediated by pulmonary CD8+ T cell infiltration. Thus, mucosal neutrophilic inflammation at the time of RSV exposure enhances susceptibility, revealing dynamic, time-dependent local immune responses before symptom onset and explaining the as-yet unpredictable outcomes of pathogen exposure.
Copyright © 2020, American Association for the Advancement of Science.
Conflict of interest statement
Figures


Comment in
-
Making a bed for viral infections.Science. 2020 Oct 9;370(6513):166-167. doi: 10.1126/science.abe3685. Science. 2020. PMID: 33033202 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

