Hydrojet-based delivery of footprint-free iPSC-derived cardiomyocytes into porcine myocardium
- PMID: 33033281
- PMCID: PMC7546722
- DOI: 10.1038/s41598-020-73693-x
Hydrojet-based delivery of footprint-free iPSC-derived cardiomyocytes into porcine myocardium
Abstract
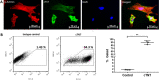
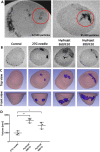
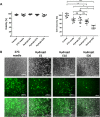
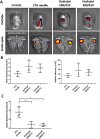
The reprogramming of patient´s somatic cells into induced pluripotent stem cells (iPSCs) and the consecutive differentiation into cardiomyocytes enables new options for the treatment of infarcted myocardium. In this study, the applicability of a hydrojet-based method to deliver footprint-free iPSC-derived cardiomyocytes into the myocardium was analyzed. A new hydrojet system enabling a rapid and accurate change between high tissue penetration pressures and low cell injection pressures was developed. Iron oxide-coated microparticles were ex vivo injected into porcine hearts to establish the application parameters and the distribution was analyzed using magnetic resonance imaging. The influence of different hydrojet pressure settings on the viability of cardiomyocytes was analyzed. Subsequently, cardiomyocytes were delivered into the porcine myocardium and analyzed by an in vivo imaging system. The delivery of microparticles or cardiomyocytes into porcine myocardium resulted in a widespread three-dimensional distribution. In vitro, 7 days post-injection, only cardiomyocytes applied with a hydrojet pressure setting of E20 (79.57 ± 1.44%) showed a significantly reduced cell viability in comparison to the cells applied with 27G needle (98.35 ± 5.15%). Furthermore, significantly less undesired distribution of the cells via blood vessels was detected compared to 27G needle injection. This study demonstrated the applicability of the hydrojet-based method for the intramyocardial delivery of iPSC-derived cardiomyocytes. The efficient delivery of cardiomyocytes into infarcted myocardium could significantly improve the regeneration.
Conflict of interest statement
LJ, WL, AF, LB, MDE are employees of Erbe Elektromedizin GmbH, Tuebingen. All other authors declare no competing financial and non-financial interests relevant to the submitted work.
Figures

References
-
- Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymers. 2011;3(2):740–761. doi: 10.3390/polym3020740. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

