Neutral sphingomyelinase 2 regulates inflammatory responses in monocytes/macrophages induced by TNF-α
- PMID: 33033337
- PMCID: PMC7544688
- DOI: 10.1038/s41598-020-73912-5
Neutral sphingomyelinase 2 regulates inflammatory responses in monocytes/macrophages induced by TNF-α
Abstract
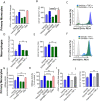
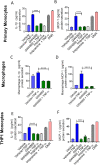
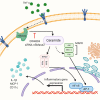
Obesity is associated with elevated levels of TNF-α and proinflammatory CD11c monocytes/macrophages. TNF-α mediated dysregulation in the plasticity of monocytes/macrophages is concomitant with pathogenesis of several inflammatory diseases, including metabolic syndrome, but the underlying mechanisms are incompletely understood. Since neutral sphingomyelinase-2 (nSMase2: SMPD3) is a key enzyme for ceramide production involved in inflammation, we investigated whether nSMase2 contributed to the inflammatory changes in the monocytes/macrophages induced by TNF-α. In this study, we demonstrate that the disruption of nSMase activity in monocytes/macrophages either by chemical inhibitor GW4869 or small interfering RNA (siRNA) against SMPD3 results in defects in the TNF-α mediated expression of CD11c. Furthermore, blockage of nSMase in monocytes/macrophages inhibited the secretion of inflammatory mediators IL-1β and MCP-1. In contrast, inhibition of acid SMase (aSMase) activity did not attenuate CD11c expression or secretion of IL-1β and MCP-1. TNF-α-induced phosphorylation of JNK, p38 and NF-κB was also attenuated by the inhibition of nSMase2. Moreover, NF-kB/AP-1 activity was blocked by the inhibition of nSMase2. SMPD3 was elevated in PBMCs from obese individuals and positively corelated with TNF-α gene expression. These findings indicate that nSMase2 acts, at least in part, as a master switch in the TNF-α mediated inflammatory responses in monocytes/macrophages.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

