SHP2 is a multifunctional therapeutic target in drug resistant metastatic breast cancer
- PMID: 33033382
- PMCID: PMC7714690
- DOI: 10.1038/s41388-020-01488-5
SHP2 is a multifunctional therapeutic target in drug resistant metastatic breast cancer
Abstract
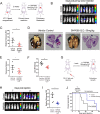
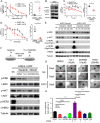
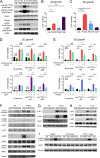
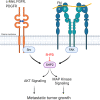
Metastatic breast cancer (MBC) is an extremely recalcitrant disease capable of bypassing current targeted therapies via engagement of several growth promoting pathways. SH2 containing protein tyrosine phosphatase-2 (SHP2) is an oncogenic phosphatase known to facilitate growth and survival signaling downstream of numerous receptor inputs. Herein, we used inducible genetic depletion and two distinct pharmacological inhibitors to investigate the therapeutic potential of targeting SHP2 in MBC. Cells that acquired resistance to the ErbB kinase inhibitor, neratinib, displayed increased phosphorylation of SHP2 at the Y542 activation site. In addition, higher levels of SHP2 phosphorylation, but not expression, were associated with decreased survival of breast cancer patients. Pharmacological inhibition of SHP2 activity blocked ERK1/2 and AKT signaling generated from exogenous stimulation with FGF2, PDGF, and hGF and readily prevented MBC cell growth induced by these factors. SHP2 was also phosphorylated upon engagement of the extracellular matrix (ECM) via focal adhesion kinase. Consistent with the potential of SHP2-targeted compounds as therapeutic agents, the growth inhibitory property of SHP2 blockade was enhanced in ECM-rich 3D culture environments. In vivo blockade of SHP2 in the adjuvant setting decreased pulmonary metastasis and extended the survival of systemic tumor-bearing mice. Finally, inhibition of SHP2 in combination with FGFR-targeted kinase inhibitors synergistically blocked the growth of MBC cells. Overall, our findings support the conclusion that SHP2 constitutes a shared signaling node allowing MBC cells to simultaneously engage a diversity of growth and survival pathways, including those derived from the ECM.
Conflict of interest statement
ZYZ is a co-founder and serves on the scientific advisory board of Tyligand Bioscience.
Figures



References
-
- Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. - PubMed
-
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. - PubMed
-
- Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–7. - PubMed
-
- Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

