Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies
- PMID: 33033554
- PMCID: PMC7524692
- DOI: 10.4252/wjsc.v12.i9.897
Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies
Abstract
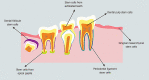
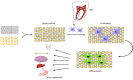
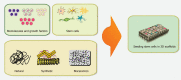
Dental stem cells (DSCs) are self-renewable cells that can be obtained easily from dental tissues, and are a desirable source of autologous stem cells. The use of DSCs for stem cell transplantation therapeutic approaches is attractive due to their simple isolation, high plasticity, immunomodulatory properties, and multipotential abilities. Using appropriate scaffolds loaded with favorable biomolecules, such as growth factors, and cytokines, can improve the proliferation, differentiation, migration, and functional capacity of DSCs and can optimize the cellular morphology to build tissue constructs for specific purposes. An enormous variety of scaffolds have been used for tissue engineering with DSCs. Of these, the scaffolds that particularly mimic tissue-specific micromilieu and loaded with biomolecules favorably regulate angiogenesis, cell-matrix interactions, degradation of extracellular matrix, organized matrix formation, and the mineralization abilities of DSCs in both in vitro and in vivo conditions. DSCs represent a promising cell source for tissue engineering, especially for tooth, bone, and neural tissue restoration. The purpose of the present review is to summarize the current developments in the major scaffolding approaches as crucial guidelines for tissue engineering using DSCs and compare their effects in tissue and organ regeneration.
Keywords: Angiogenesis; Biomolecules; Cell transplantation; Neural crest; Regenerative medicine; Tissue engineering.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There is no conflict of interest.
Figures
References
-
- Caplan AI. Are All Adult Stem Cells The Same? Regen Eng Transl Med. 2015;1:4–10.
Publication types
LinkOut - more resources
Full Text Sources

