Noble gas endohedral fullerenes
- PMID: 33033593
- PMCID: PMC7500087
- DOI: 10.1039/d0sc02507k
Noble gas endohedral fullerenes
Abstract
This review focuses on the available experimental and theoretical investigations on noble gas (Ng) endohedral fullerenes, addressing essential questions related to the mutual effects that confinement of one or more Ng atoms induces on the electronic structure, bonding, and different properties of fullerenes. It also summarizes the different contributions to the mechanisms of formation and decomplexation, the reactivity towards Diels-Alder cycloaddition reactions, the chemical bonding situation of Ng endohedral fullerenes, and the interactions that dominate within these systems.
This journal is © The Royal Society of Chemistry 2020.
Figures
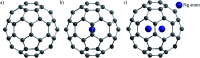
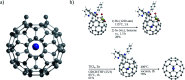

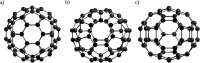
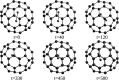
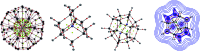

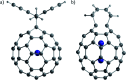
References
-
- Kroto H. W., Heath J. R., O'Brien S. C., Curl R. F., Smalley R. E. Nature. 1985;318:162–163.
-
- Sprang H., Mahlkow A., Campbell E. E. B. Chem. Phys. Lett. 1994;227:91–97.
-
- Heath J. R., O'Brien S. C., Zhang Q., Liu Y., Curl R. F., Tittel F. K., Smalley R. E. J. Am. Chem. Soc. 1985;107:7779–7780.
-
- Chai Y., Guo T., Jin C., Haufler R. E., Felipe Chibante L. P., Fure J., Wang L., Michael Alford J., Smalley R. E. J. Phys. Chem. 1991;95:7564–7568.
-
- Popov A. A., Yang S., Dunsch L. Chem. Rev. 2013;113:5989–6113. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

