Progressive Reinvention or Destination Lost? Half a Century of Cardiovascular Tissue Engineering
- PMID: 33033720
- PMCID: PMC7509093
- DOI: 10.3389/fcvm.2020.00159
Progressive Reinvention or Destination Lost? Half a Century of Cardiovascular Tissue Engineering
Abstract
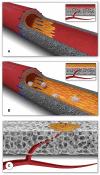
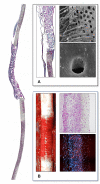
The concept of tissue engineering evolved long before the phrase was forged, driven by the thromboembolic complications associated with the early total artificial heart programs of the 1960s. Yet more than half a century of dedicated research has not fulfilled the promise of successful broad clinical implementation. A historical account outlines reasons for this scientific impasse. For one, there was a disconnect between distinct eras each characterized by different clinical needs and different advocates. Initiated by the pioneers of cardiac surgery attempting to create neointimas on total artificial hearts, tissue engineering became fashionable when vascular surgeons pursued the endothelialisation of vascular grafts in the late 1970s. A decade later, it were cardiac surgeons again who strived to improve the longevity of tissue heart valves, and lastly, cardiologists entered the fray pursuing myocardial regeneration. Each of these disciplines and eras started with immense enthusiasm but were only remotely aware of the preceding efforts. Over the decades, the growing complexity of cellular and molecular biology as well as polymer sciences have led to surgeons gradually being replaced by scientists as the champions of tissue engineering. Together with a widening chasm between clinical purpose, human pathobiology and laboratory-based solutions, clinical implementation increasingly faded away as the singular endpoint of all strategies. Moreover, a loss of insight into the healing of cardiovascular prostheses in humans resulted in the acceptance of misleading animal models compromising the translation from laboratory to clinical reality. This was most evident in vascular graft healing, where the two main impediments to the in-situ generation of functional tissue in humans remained unheeded-the trans-anastomotic outgrowth stoppage of endothelium and the build-up of an impenetrable surface thrombus. To overcome this dead-lock, research focus needs to shift from a biologically possible tissue regeneration response to one that is feasible at the intended site and in the intended host environment of patients. Equipped with an impressive toolbox of modern biomaterials and deep insight into cues for facilitated healing, reconnecting to the "user needs" of patients would bring one of the most exciting concepts of cardiovascular medicine closer to clinical reality.
Keywords: cardiovascular tissue engineering; clinical needs; history; misleading animal models; refocusing translation.
Copyright © 2020 Zilla, Deutsch, Bezuidenhout, Davies and Pennel.
Figures

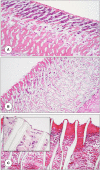
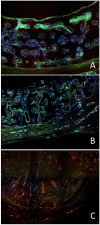
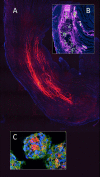
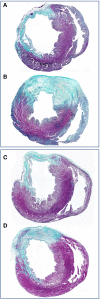





References
-
- Cooley DA, Mahaffey DE, De Bakey ME. Total excision of the aortic arch for aneurysm. Surg Gynecol Obstet. (1955) 101:667–72. - PubMed
-
- Kirklin JW, Dushane JW, Patrick RT, Donald DE, Hetzel PS, Harshbarger HG. Intracardiac surgery with the aid of a mechanical pump-oxygenator system (gibbon type): report of eight cases. Proc Staff Meet Mayo Clin. (1955) 30:201–6. - PubMed
-
- Melrose DG. A heart-lung machine for use in man. J Physiol. (1955) 127:51–3. - PubMed
-
- Melrose DG. The principles of heart-lung machines. Lect Sci Basis Med. (1956) 6:85–99. - PubMed
-
- Kirby CK, Johnson J. Aortic valve replacement for acquired aortic stenosis and insufficiency. Trans Am Soc Artif Intern Organs. (1961) 7:306–17. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

