Discordant DNA mismatch repair protein status between synchronous or metachronous gastrointestinal carcinomas: frequency, patterns, and molecular etiologies
- PMID: 33033905
- PMCID: PMC8032798
- DOI: 10.1007/s10689-020-00210-4
Discordant DNA mismatch repair protein status between synchronous or metachronous gastrointestinal carcinomas: frequency, patterns, and molecular etiologies
Abstract
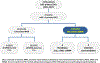
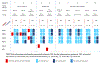
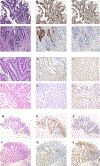
The widespread use of tumor DNA mismatch repair (MMR) protein immunohistochemistry in gastrointestinal tract (GIT) carcinomas has unveiled cases where the MMR protein status differs between synchronous/metachronous tumors from the same patients. This study aims at examining the frequency, patterns and molecular etiologies of such inter-tumoral MMR discordances. We analyzed a cohort of 2159 colorectal cancer (CRC) patients collected over a 5-year period and found that 1.3% of the patients (27/2159) had ≥ 2 primary CRCs, and 25.9% of the patients with ≥ 2 primary CRCs (7/27) exhibited inter-tumoral MMR discordance. We then combined the seven MMR-discordant CRC patients with three additional MMR-discordant GIT carcinoma patients and evaluated their discordant patterns and associated molecular abnormalities. The 10 patients consisted of 3 patients with Lynch syndrome (LS), 1 with polymerase proofreading-associated polyposis (PAPP), 1 with familial adenomatous polyposis (FAP), and 5 deemed to have no cancer disposing hereditary syndromes. Their MMR discordances were associated with the following etiologies: (1) PMS2-LS manifesting PMS2-deficient cancer at an old age when a co-incidental sporadic MMR-proficient cancer also occurred; (2) microsatellite instability-driven secondary somatic MSH6-inactivation occurring in only one-and not all-PMS2-LS associated MMR-deficient carcinomas; (3) "compound LS" with germline mutations in two MMR genes manifesting different tumors with deficiencies in different MMR proteins; (4) PAPP or FAP syndrome-associated MMR-proficient cancer co-occurring metachronously with a somatic MMR-deficient cancer; and (5) non-syndromic patients with sporadic MMR-proficient cancers co-occurring synchronously/metachronously with sporadic MMR-deficient cancers. Our study thus suggests that inter-tumoral MMR discordance is not uncommon among patients with multiple primary GIT carcinomas (25.9% in patients with ≥ 2 CRCs), and may be associated with widely varied molecular etiologies. Awareness of these patterns is essential in ensuring the most effective strategies in both LS detection and treatment decision-making. When selecting patients for immunotherapy, MMR testing should be performed on the tumor or tumors that are being treated.
Keywords: Colorectal cancer; Gastrointestinal tract cancer; Hereditary cancer; Lynch syndrome; MMR IHC; Mismatch repair deficiency; Mutational testing.
Figures


Similar articles
-
The Clinical Outcomes Among Patients Under 60 Years Old with Lynch Syndrome: Variations Based on Different Mutation Patterns.Int J Mol Sci. 2025 Apr 4;26(7):3383. doi: 10.3390/ijms26073383. Int J Mol Sci. 2025. PMID: 40244260 Free PMC article. Review.
-
The coding microsatellite mutation profile of PMS2-deficient colorectal cancer.Exp Mol Pathol. 2021 Oct;122:104668. doi: 10.1016/j.yexmp.2021.104668. Epub 2021 Jul 22. Exp Mol Pathol. 2021. PMID: 34302852
-
Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers.Cancer. 2024 Feb 1;130(3):385-399. doi: 10.1002/cncr.35030. Epub 2023 Sep 26. Cancer. 2024. PMID: 37751191 Free PMC article. Review.
-
Clinicopathologic Comparison of Lynch Syndrome-associated and "Lynch-like" Endometrial Carcinomas Identified on Universal Screening Using Mismatch Repair Protein Immunohistochemistry.Am J Surg Pathol. 2016 Feb;40(2):155-65. doi: 10.1097/PAS.0000000000000544. Am J Surg Pathol. 2016. PMID: 26523542
-
Mismatch Repair Proficient Colorectal Adenocarcinoma in Two Patients With Lynch Syndrome.Clin Genet. 2025 Apr;107(4):469-474. doi: 10.1111/cge.14670. Epub 2024 Dec 11. Clin Genet. 2025. PMID: 39660603
Cited by
-
Treatment of Microsatellite-Unstable Rectal Cancer in Sporadic and Hereditary Settings.Clin Colon Rectal Surg. 2023 Aug 11;37(4):233-238. doi: 10.1055/s-0043-1770717. eCollection 2024 Jul. Clin Colon Rectal Surg. 2023. PMID: 38882941 Free PMC article. Review.
-
Primary Clonal Loss of Mismatch Repair Protein on Immunohistochemistry: A Pattern of Abnormality That Warrants Genetic Workup.JCO Precis Oncol. 2022 Jun;6:e2200111. doi: 10.1200/PO.22.00111. JCO Precis Oncol. 2022. PMID: 35700411 Free PMC article. No abstract available.
-
Minimal residual disease monitoring via ctDNA: a case report of Lynch syndrome with synchronous colorectal cancer and review of literature.J Gastrointest Oncol. 2024 Jun 30;15(3):1341-1347. doi: 10.21037/jgo-24-81. Epub 2024 Jun 19. J Gastrointest Oncol. 2024. PMID: 38989405 Free PMC article.
-
Inoperable de novo metastatic colorectal cancer with primary tumour in situ: Evaluating discordant responses to upfront systemic therapy of the primary tumours and metastatic sites and complications arising from primary tumours (experiences from an Irish Cancer Centre).Mol Clin Oncol. 2022 Feb;16(2):40. doi: 10.3892/mco.2021.2472. Epub 2021 Dec 21. Mol Clin Oncol. 2022. PMID: 35003738 Free PMC article.
References
-
- Samowitz WS. Evaluation of colorectal cancers for Lynch syndrome: practical molecular diagnostics for surgical pathologists. Mod Pathol 2015;28 Suppl1:S109–13. - PubMed
-
- Rybak C, Hall MJ.Interpretation of genetic testing for lynch syndrome in patients with putative familial colorectal cancer. J Natl Compr Canc Netw 2011;9:1311–20. - PubMed
-
- Yurgelun MB, Hampel H. Recent Advances in Lynch Syndrome: Diagnosis, Treatment, and Cancer Prevention. Am Soc Clin Oncol Educ Book 2018;38:101–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

