Temperature Screening for SARS-CoV-2 in Nursing Homes: Evidence from Two National Cohorts
- PMID: 33034046
- PMCID: PMC7675320
- DOI: 10.1111/jgs.16876
Temperature Screening for SARS-CoV-2 in Nursing Homes: Evidence from Two National Cohorts
Abstract
Background/objectives: Infection screening tools classically define fever as 38.0°C (100.4°F). Frail older adults may not mount the same febrile response to systemic infection as younger or healthier individuals. We evaluate temperature trends among nursing home (NH) residents undergoing diagnostic SARS-CoV-2 testing and describe the diagnostic accuracy of temperature measurements for predicting test-confirmed SARS-CoV-2 infection.
Design: Retrospective cohort study evaluating diagnostic accuracy of pre-SARS-CoV-2 testing temperature changes.
Setting: Two separate NH cohorts tested diagnostically (e.g., for symptoms) for SARS-CoV-2. PARTICIPANTS Veterans residing in Veterans Affairs (VA) managed NHs and residents in a private national chain of community NHs.
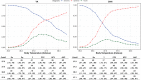
Measurements: For both cohorts, we determined the sensitivity, specificity, and Youden's index with different temperature cutoffs for SARS-CoV-2 polymerase chain reaction results.
Results: The VA cohort consisted of 1,301 residents in 134 facilities from March 1, 2020, to May 14, 2020, with 25% confirmed for SARS-CoV-2. The community cohort included 3,368 residents spread across 282 facilities from February 18, 2020, to June 9, 2020, and 42% were confirmed for SARS-CoV-2. The VA cohort was younger, less White, and mostly male. A temperature testing threshold of 37.2°C has better sensitivity for SARS-CoV-2, 76% and 34% in the VA and community NH, respectively, versus 38.0°C with 43% and 12% sensitivity, respectively.
Conclusion: A definition of 38.0°C for fever in NH screening tools should be lowered to improve predictive accuracy for SARS-CoV-2 infection. Stakeholders should carefully consider the impact of adopting lower testing thresholds on testing availability, cost, and burden on staff and residents. Temperatures alone have relatively low sensitivity/specificity, and we advocate any threshold be used as part of a screening tool, along with other signs and symptoms of infection.
Keywords: COVID-19; SARS-CoV-2; aged 80 and older; nursing homes; temperature.
© 2020 The American Geriatrics Society.
Conflict of interest statement
Kevin W. McConeghy reports grants from Sanofi Pasteur and Pfizer outside of the submitted work. Elizabeth White reports support from the National Institute on Aging and employment by the PACE organization of Rhode Island. Vince Mor reports grants from Sanofi Pasteur, Pfizer, and Seqirus related to vaccination in nursing homes. He also receives fees for chairing the Scientific Advisory Committee of NaviHealth, a post‐acute care company. Stefan Gravenstein reports grants and personal fees from Sanofi Pasteur and Pfizer, and consulting or speaker fees from Catapult Consultants, GlaxoSmithKline, Healthcentric Advisors, Janssen, Merck, Novartis, Pfizer, and Longeveron related to vaccines or nursing home care quality. The remaining authors have declared no conflicts of interest for this article.
Figures

References
-
- Kaiser Family Foundation . State data and policy actions to address coronavirus. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actio.... Accessed June 25, 2020.
-
- Centers for Disease Control and Prevention . Testing guidelines for nursing homes. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html. Accessed June 26, 2020.
-
- Gou FX, Zhang XS, Yao JX, et al. Epidemiological characteristics of COVID‐19 in Gansu province [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:E032. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

