Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial
- PMID: 33034095
- PMCID: PMC7675325
- DOI: 10.1111/joim.13185
Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial
Erratum in
-
Correction to "Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial".J Intern Med. 2024 Nov;296(5):456. doi: 10.1111/joim.20011. Epub 2024 Oct 1. J Intern Med. 2024. PMID: 39352690 Free PMC article. No abstract available.
Abstract
Background: Convalescent plasma therapy for COVID-19 relies on transfer of anti-viral antibody from donors to recipients via plasma transfusion. The relationship between clinical characteristics and antibody response to COVID-19 is not well defined. We investigated predictors of convalescent antibody production and quantified recipient antibody response in a convalescent plasma therapy clinical trial.
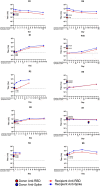
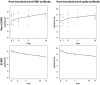
Methods: Multivariable analysis of clinical and serological parameters in 103 confirmed COVID-19 convalescent plasma donors 28 days or more following symptom resolution was performed. Mixed-effects regression models with piecewise linear trends were used to characterize serial antibody responses in 10 convalescent plasma recipients with severe COVID-19.
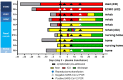
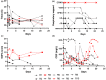
Results: Donor antibody titres ranged from 0 to 1 : 3892 (anti-receptor binding domain (RBD)) and 0 to 1 : 3289 (anti-spike). Higher anti-RBD and anti-spike titres were associated with increased age, hospitalization for COVID-19, fever and absence of myalgia (all P < 0.05). Fatigue was significantly associated with anti-RBD (P = 0.03). In pairwise comparison amongst ABO blood types, AB donors had higher anti-RBD and anti-spike than O donors (P < 0.05). No toxicity was associated with plasma transfusion. Non-ECMO recipient anti-RBD antibody titre increased on average 31% per day during the first three days post-transfusion (P = 0.01) and anti-spike antibody titre by 40.3% (P = 0.02).
Conclusion: Advanced age, fever, absence of myalgia, fatigue, blood type and hospitalization were associated with higher convalescent antibody titre to COVID-19. Despite variability in donor titre, 80% of convalescent plasma recipients showed significant increase in antibody levels post-transfusion. A more complete understanding of the dose-response effect of plasma transfusion amongst COVID-19-infected patients is needed.
Keywords: COVID-19; antibody titre; convalescent plasma.
© 2020 The Association for the Publication of the Journal of Internal Medicine.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007605/HL/NHLBI NIH HHS/United States
- National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051
- Department of Surgery University of Chicago
- T32 AI007090/AI/NIAID NIH HHS/United States
- 75N93019C00051/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

